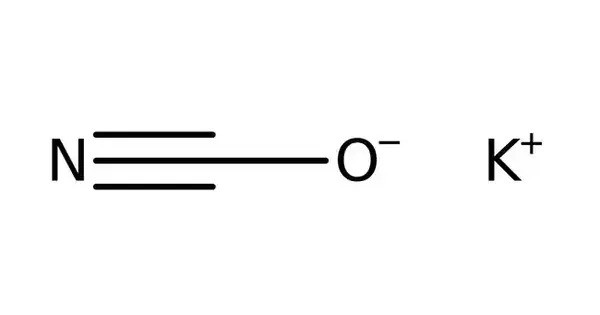Potassium cyanate is an inorganic compound with the formula KOCN (sometimes denoted KCNO). It is a colourless solid. It is a white, crystalline substance that is soluble in water and alcohol. It is often used as a reagent in chemical synthesis, especially in organic chemistry. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.
Properties
- Chemical formula: KOCN
- Molar mass: 81.1151 g/mol
- Appearance: white, crystalline powder
- Density: 2.056 g/cm3
- Melting point: 315 °C (599 °F; 588 K)
- Boiling point: ~ 700 °C (1,292 °F; 973 K) decomposes
- Solubility in water: 75 g/100 mL
- Solubility: very slightly soluble in alcohol
Chemical Behavior
Potassium cyanate is a strong nucleophile and can participate in reactions involving the cyanate group (–OCN).
It can hydrolyze in water, forming cyanate ions and potassium hydroxide:
𝐾𝑂𝐶𝑁 → 𝐾+ + 𝑂𝐶𝑁–
Uses
The potassium and sodium salts can be used interchangeably for the majority of applications. Potassium cyanate is often preferred to the sodium salt, which is less soluble in water and less readily available in pure form.
Potassium cyanate is used as a basic raw material for various organic syntheses, including, urea derivatives, semicarbazides, carbamates and isocyanates. For example, it is used to prepare the drug hydroxyurea. It is also used for the heat treatment of metals (e.g., Ferritic nitrocarburizing).
- Synthesis of Ureides: Potassium cyanate can be used to synthesize compounds like urea and is involved in the production of other nitrogen-containing organic compounds.
- Organic Synthesis: It serves as a precursor for cyanate esters, which are used in advanced polymeric materials.
- Agriculture: It is sometimes used as a fertilizer due to its nitrogen content.
- Chemical Reactions: It is employed in chemical reactions like the preparation of isocyanates, used in plastics, resins, and foams.
Safety Considerations
Potassium cyanate, like cyanide compounds, is highly toxic if ingested, inhaled, or absorbed through the skin. It can release toxic fumes when heated to decomposition, making it important to handle with care in well-ventilated areas and with appropriate safety equipment.