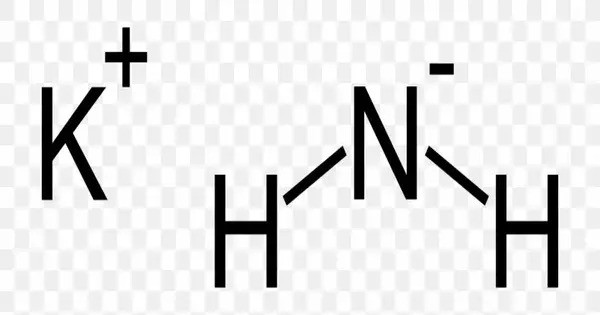
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base. It is a highly reactive and useful compound in synthetic chemistry, especially in reactions requiring a strong base. It is often handled in controlled environments to avoid hazardous reactions with moisture or air.
Properties
Potassium amide is usually a white or pale-yellow solid. It is soluble in liquid ammonia and may react vigorously with water to produce potassium hydroxide (KOH) and ammonia gas (NH₃). It is highly reactive and will readily deprotonate weak acids, such as alcohols and even hydrocarbons, making it a strong base in organic synthesis.
- Chemical formula: KNH2
- Molar mass: 55.121 g·mol−1
- Appearance: white solid
- Odor: ammonia-like
- Density: 1.57 g/cm 3
- Melting point: 338 °C (640 °F; 611 K)
- Solubility in water: reacts
- Solubility: ammonia: 3.6 g/(100 mL)
Production
Potassium amide is produced by the reaction of ammonia with potassium. The reaction typically requires a catalyst.
Structure
Traditionally KNH2 is viewed as a simple salt, but it has significant covalent character and is highly aggregated in ammonia solution. The compound has been characterized by X-ray crystallography as the solvent-free form as well as the mono- and diammonia solvates. In KNH2·2NH3, the potassium centers are each bonded to two amido ligands and four ammonia ligands, all six of which bridge to adjacent potassium centers. The result is a chain of hexacoordinate potassium ions. The K–NH2 distances are 2.7652(11) whereas the K–NH3 distances are respectively 2.9234(11) and 3.0698(11) Å.
Uses
- Synthesis of organic compounds: Potassium amide is used as a base in the preparation of various organic compounds, such as nitriles and imines. It can also be used in the formation of carbon-carbon bonds through nucleophilic substitution or elimination reactions.
- Metal amides: Potassium amide can be involved in the synthesis of other metal amides.
Safety
Due to its high reactivity, particularly with water, potassium amide should be handled with care, ideally in anhydrous conditions, and away from moisture or other reactive materials.