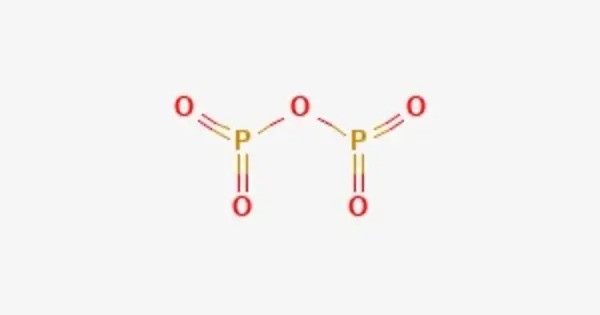
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). It is a white, crystalline solid at room temperature. This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent. It is highly hygroscopic, meaning it readily absorbs water from the atmosphere. When exposed to moisture, it reacts vigorously to form phosphoric acid (H₃PO₄).
Phosphorus pentoxide is a potent dehydrating agent due to its ability to remove water molecules from other substances. This property makes it useful in chemical synthesis and in dehydration reactions. When it reacts with water, it forms phosphoric acid, which is a weak acid but can affect pH levels in solutions significantly.
Properties
- Chemical formula: P4O10
- Molar mass: 283.9 g mol−1
- Appearance: White powder, Very deliquescent
- Odor: Odorless
- Density: 2.39 g/cm3
- Melting point: 340 °C (644 °F; 613 K)
- Boiling point: 360 °C (sublimes)
- Solubility in water: exothermic hydrolysis
- Vapor pressure: 1 mmHg @ 385 °C (stable form)
Preparation
P4O10 is prepared by burning white phosphorus with a sufficient supply of oxygen:
P4 + 5 O2 → P4O10
The dehydration of phosphoric acid to give phosphorus pentoxide is not possible, as on heating it forms various polyphosphates but will not dehydrate sufficiently to form P4O10. This reaction makes phosphorus pentoxide a useful dehydrating agent in various chemical processes. In addition to its role in dehydration reactions, it is used in laboratories and industry for its ability to absorb moisture and in the synthesis of phosphoric acid and other phosphorus-containing compounds.
Phosphorus pentoxide is not typically found in nature but is instead produced synthetically, often from the oxidation of phosphorus trichloride or from the direct combustion of phosphorus. Its primary applications are in the chemical industry rather than in everyday use.
Natural Occurrence
Phosphorus pentoxide is not found in its pure form in nature. Instead, it is derived from phosphorus-containing minerals such as apatite, which is a major source of phosphorus for commercial production.
Applications
Phosphorus pentoxide is a potent dehydrating agent as indicated by the exothermic nature of its hydrolysis producing phosphoric acid:
P4O10 + 6 H2O → 4 H3PO4 (–177 kJ)
However, its utility for drying is limited somewhat by its tendency to form a protective viscous coating that inhibits further dehydration by unspent material. A granular form of P4O10 is used in desiccators.
Phosphorus pentoxide is used extensively in the production of phosphoric acid and in various chemical syntheses. It’s also used as a desiccant in certain applications due to its strong dehydrating properties. In the lab, it is used as a drying agent and a reagent in organic synthesis. It helps in removing water from various chemical reactions and processes.