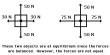
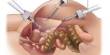
Phosphogypsum (PG) is a calcium sulfate hydrate that forms as a byproduct of fertilizer manufacture from phosphate rock, namely phosphoric acid. It is primarily made of gypsum (CaSO4•2H2O). It is a byproduct of the phosphate fertilizer industry, specifically the manufacture of phosphoric acid. When phosphate rock is processed with sulfuric acid to yield phosphoric acid, phosphogypsum is produced as a solid waste. It is predominantly composed of calcium sulfate dihydrate (gypsum), with impurities including fluoride, phosphate, and organic materials.
Here are some key points about phosphogypsum:
- Composition: It is mostly composed of calcium sulfate dihydrate (CaSO4·2H2O), similar to natural gypsum, but with varying impurities depending on the source and processing methods.
- Environmental Concerns: Phosphogypsum is radioactive due to the presence of naturally occurring radionuclides like uranium, thorium, and radium. Its radioactivity levels can vary, and proper management is required to prevent environmental contamination.
- Disposal and Use: Due to its radioactivity, regulations often restrict its direct use or disposal. However, efforts are made to find beneficial uses, such as in construction materials (e.g., drywall), agriculture (as a soil amendment), or in landfill construction.
- Global Production: Large quantities of phosphogypsum are produced globally each year, primarily in countries with significant phosphate rock processing industries.
- Regulation: The management and disposal of phosphogypsum are regulated in many countries to minimize environmental and health risks associated with its radioactivity and chemical composition.
Although gypsum is widely used in the construction industry, phosphogypsum is rarely used and is stored indefinitely due to its low radioactivity caused by the presence of naturally occurring uranium (U) and thorium (Th), as well as their daughter isotopes radium (Ra), radon (Rn), and polonium (Po).
On the other hand, it contains various useful components, including calcium sulphates and elements like silicon, iron, titanium, magnesium, aluminum, and manganese. However, long-term storage of phosphogypsum is problematic. Each ton of phosphoric acid produced yields approximately five tons of phosphogypsum. The world’s annual phosphogypsum production is estimated to be between 100 and 280 million metric tons.