Phosphine (PH3), also known as hydrogen phosphide, is a colorless, combustible gas that is heavier than air and has a stench that has been compared to that of decomposing fish. Due to the presence of substituted phosphine and diphosphane, technical grade samples have a very terrible odor that smells like rotting fish (P2H4). It’s made when a strong base or hot water reacts with white phosphorus, or when water reacts with calcium phosphide (Ca3P2).
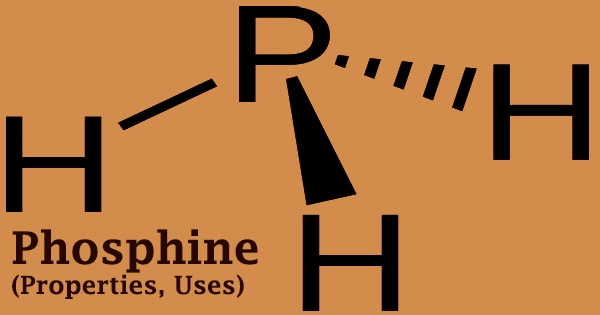
Phosphine is physically identical to ammonia (NH3), however, it is a far worse solvent and less water-soluble than ammonia. The odor threshold for commercially available phosphine is between 0.02 and 3 parts per million. When residues of other phosphorous hydrides, such as diphosphine, are present, it ignites spontaneously at a temperature of 100°F (37.8°C). Phosphine is a very poisonous respiratory toxin with a trigonal pyramidal structure that is instantly harmful to life or health at 50 ppm.
Phosphine should be treated as a pyrophoric and extremely poisonous gas for all practical purposes. At extremely low quantities, it is highly hazardous when inhaled. Long-term heating might cause containers to explode violently. Phosphine is also the generic name for a series of organophosphorus compounds with the general formula PH3-nRn, in which one or more hydrogen atoms in PH3 have been substituted by organic derivatives.

At room temperature, phosphophine is a colorless, combustible, and explosive gas with a garlic or rotten fish stench. Small quantities are produced spontaneously when organic stuff decomposes. In 1783, Philippe Gengembre (1764–1838), a Lavoisier student, produced phosphine for the first time by heating white phosphorus in an aqueous solution of potash (potassium carbonate). Phosphine is somewhat soluble in water, stable at ambient temperature, and begins to degrade about 707°F (375°C), with total disintegration occurring around 1100°F (593°C).
Derivatives of phosphine are organic compounds having phosphorus-carbon or hydrogen bonds; one, two, or three hydrogen atoms have been substituted by organic combining groups in primary, secondary, and tertiary phosphines, respectively. Phosphine is a pyrophoric compound that burns readily in the air. Strong oxidizing agents, halogens, and nitric acid are incompatible with it. At room temperature, it is a volatile and explosive gas. Phosphine decomposes into hazardous gases, particularly phosphorus oxides when heated or burned.
In 1857, the term “phosphine” was first applied to organophosphorus compounds that were comparable to organic amines (NR3). By 1865, the gas PH3 had been given the name “phosphine” (or earlier). Phosphine is utilized in the semiconductor and plastics sectors, as well as in the manufacture of a flame retardant and as a crop insecticide. Common oxidizers like potassium permanganate and sodium hypochlorite may easily oxidize it. Unlike arsine, it will react with alkalis in some way.
The simplest phosphine is phosphophane, which is made up of a single phosphorus atom with three hydrogens connected. It functions as both a carcinogen and a fumigant pesticide. It interacts aggressively with air, oxygen, oxidants including chlorine and nitrogen oxides, metal nitrates, halogens, and other hazardous compounds, posing a risk of fire and explosion. Because of the non-polar PH bonds, hosphine dissolves more readily in non-polar solvents than in water. Although it is theoretically amphoteric in water, it has little acid and base activity.
As a result, methylphosphine (CH3PH2) is a primary phosphine in which the methyl group (CH3) replaces one of the phosphine’s hydrogen atoms. Phosphine is naturally created in tiny amounts in marshy places, particularly in moist graveyards, as a result of bacterial degradation of phosphorus-containing animal and vegetable debris. It’s a phosphine and a mononuclear parent hydride from the phosphane family. It is both a conjugate base of phosphonium and a conjugate acid of phosphanide.
Phosphine is utilized in the production of phosphonium halides and a range of organic preparations. It’s frequently utilized as a doping agent for n-type semiconductors (in gas mixtures) and as a pure gas in the production of light-emitting diodes. It’s also utilized as a grain fumigant at low quantities. It may be produced industrially by reacting white phosphorus with sodium or potassium hydroxide, yielding potassium or sodium hypophosphite as a by-product.
3 KOH + P4 + 3 H2O → 3 KH2PO2 + PH3
Substitution processes using easily accessible phosphorus trichloride are commonly used to make organic phosphine derivatives (PCl3). Because environmental systems lack known reducing agents of sufficient strength to directly convert phosphate to phosphine, the most plausible source is phosphate reduction in decaying organic matter, potentially via partial reductions and disproportionations. Phosphine is employed as an insecticide, as well as a rodenticide, in the fumigation of cereals, animal feed, and leaf-stored tobacco.
Phosphine can also be made by reducing a phosphorus trichloride solution with lithium aluminum hydride in dry ether at room temperature. A dropping funnel is used to add the latter’s solution to a phosphorus trichloride solution in the dry ether in a water bath. Due to the Montreal Protocol’s phase-out of the formerly popular fumigant methyl bromide in some countries, phosphine is the only extensively used, cost-effective, swiftly acting fumigant that does not leave residues on the stored goods.
Information Sources: