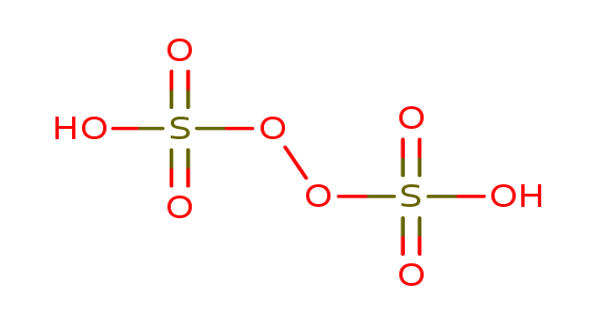
Peroxydisulfuric acid is a sulfur oxoacid. It is an inorganic compound with a chemical formula H2S2O8. It is the conjugate acid of peroxydisulfate. Also called Marshall’s acid after its inventor Professor Hugh Marshall, it is a sulfur oxoacid. In structural terms, it can be written HO3SOOSO3H. It is a solid that melts at 65°C. It is a colorless solid and its salts are strong oxidizing agents, and the acid is not very stable.
The acid and its salts are such strong oxidizing agents that combustible materials can be ignited by them. It contains sulfur in its +6 oxidation state and a peroxide group. The usual salts that are widely used as oxidizing agents are the sodium, potassium, and ammonium salts. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents.
Preparation
It can be generated from potassium or ammonium persulfate in acidic solution. It is an anhydride of sulphuric and peroxydisulfuric acid and may be prepared by oxidation of oleum with ozone or hydrogen peroxide.
Peroxydisulfuric Acid can be prepared by adding the concentrated sulfuric acid to the sodium persulfate compound. Also, this acid can be prepared using chlorosulfuric acid, including a hydrogen peroxide reaction. The chemical equation for the same can be given as follows:
2ClSO3H + H2O → H2S2O8+ 2HCl
The solid form is much difficult to obtain and is more often encountered as a solution.
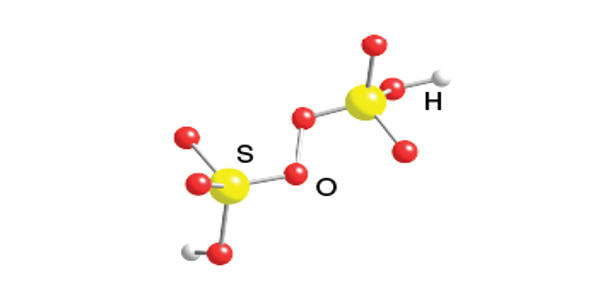
Synthesis
The acid is prepared by the reaction of chlorosulfuric acid with hydrogen peroxide:
2ClSO3H + H2O2 → H2S2O8 + 2 HCl
Another method is the electrolysis of moderately concentrated sulfuric acid (60-70%) with platinum electrodes at high current density and voltage:
H2SO4 + H2O → H3O+ + HSO4– (dissociation of sulfuric acid)
2HSO4– → H2S2O8 + 2e– (E0 = +2.4V) (bisulfate oxidation)
2H2SO4 → H2S2O8 + H2 (overall reaction)
3H2O → O3 + 6H+ (ozone produced as a side product)
Peroxydisulfuric Acid dissolves in water forms hydrogen peroxide and sulphuric acid. The chemical equation is given below.
H2S2O8 + 2H2O → H O2 + 2H2SO4
Peroxydisulfuric Acid reacts with silver nitrate in aqueous medium forms silver oxide, sulphuric acid and nitric acid.
Uses
- Use of peroxydisulfuric acid and its salts as a source of hydrogen peroxide opened the way for large-scale production of sulphuric acid.
- Used as a hypo eliminator in photography.
- Used as a strong oxidant but the quantity of the oxidizing agent used can be varied in accordance to the desired reaction rate.
Information Source: