Catalysts can, in fact, help to control methane emissions in natural gas engines. Methane is a powerful greenhouse gas, and reducing its emissions is critical for mitigating climate change and improving air quality.
In a recent study, a catalyst containing a single or a few palladium atoms removed 90% of unburned methane from natural gas engine exhaust at low temperatures. While more research is needed, advances in single atom catalysis have the potential to reduce methane exhaust emissions, one of the worst greenhouse gases that traps heat at approximately 25 times the rate of carbon dioxide.
A research collaboration between Washington State University and SLAC National Accelerator Laboratory reported in the journal Nature Catalysis that the single-atom catalyst could remove methane from engine exhaust at lower temperatures, less than 350 degrees Celsius (662 degrees Fahrenheit), while maintaining reaction stability at higher temperatures.
“It’s almost a self-modulating process that miraculously overcomes the challenges that people have been fighting — low temperature inactivity and high temperature instability,” said Yong Wang, Regents Professor in WSU’s Gene and Linda Voiland School of Chemical Engineering and Bioengineering and corresponding author on the paper.
There’s a big drive towards using natural gas, but when you use it for combustion engines, there will always be unburnt natural gas from the exhaust, and you have to find a way to remove that. If not, you cause more severe global warming.
Frank Abild-Pedersen
Natural gas engines are used in approximately 30 million to 40 million vehicles worldwide, with a strong presence in Europe and Asia. They are also used in the gas industry to power compressors that deliver natural gas to people’s homes. They produce less carbon and particulate pollution than gasoline or diesel engines and are therefore considered cleaner.
However, because their catalytic converters do not work well at low temperatures, when these natural gas-powered engines start up, they emit unburned, heat-trapping methane. Methane removal catalysts are either inefficient at low exhaust temperatures or severely degrade at high temperatures.
“There’s a big drive towards using natural gas, but when you use it for combustion engines, there will always be unburnt natural gas from the exhaust, and you have to find a way to remove that. If not, you cause more severe global warming,” said co-author Frank Abild-Pedersen, a staff scientist at SLAC National Accelerator Laboratory. “If you can remove 90% of the methane from the exhaust and keep the reaction stable, that’s tremendous.”
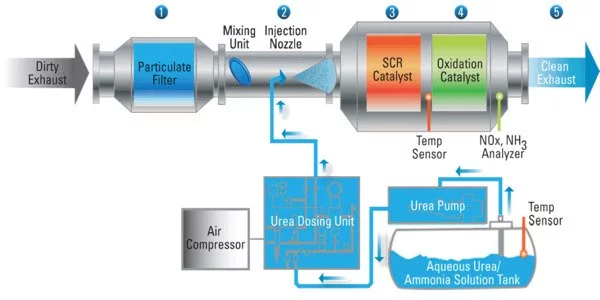
A single-atom catalyst with the active metals singly dispersed on a support also uses every atom of the expensive and precious metals, Wang added. “If you can make them more reactive, that’s the icing on the cake,” he said.
In their work, the researchers were able to show that their catalyst made from single palladium atoms on a cerium oxide support efficiently removed methane from engine exhaust, even when the engine was just starting.
They discovered that trace amounts of carbon monoxide, which are always present in engine exhaust, played an important role in dynamically forming active sites for the reaction at room temperature. Carbon monoxide aided the migration of single palladium atoms to form two- or three-atom clusters that efficiently break apart methane molecules at low temperatures.
As the exhaust temperatures rose, the sub-nanometer-sized clusters re-dispersed to single atoms, allowing the catalyst to remain thermally stable. This reversible process allows the catalyst to work effectively and uses every palladium atom throughout the engine’s operation, including when it starts cold.
“We were really able to find a way to keep the supported palladium catalyst stable and highly active, and because of the diverse expertise across the team, we were able to understand why this was occurring,” said Christopher Tassone, a staff scientist at SLAC National Accelerator Laboratory and co-author on the paper.
The researchers are working to improve catalyst technology. They want to know why palladium behaves one way while other precious metals, such as platinum, behave differently. The research has a long way to go before it can be installed in a car, but the researchers are working with industry partners and the Pacific Northwest National Laboratory to bring the work closer to commercialization.