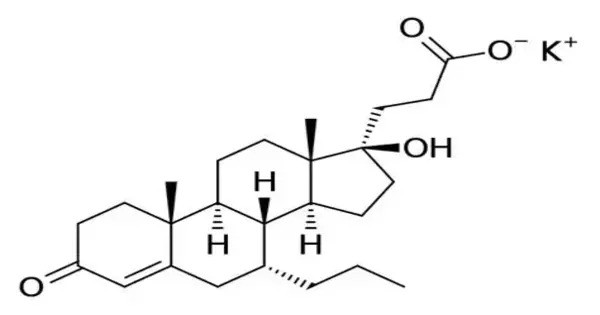
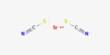
Oxprenoate potassium (developmental code name RU-28318) is a synthetic steroidal antimineralocorticoid which was never marketed. It is a synthetic steroidal compound primarily known as a progestin—a drug that mimics the effects of the natural hormone progesterone. The affinities of oxprenoate potassium for the steroid hormone receptors have been reported. It is a potassium salt of oxprenoic acid and has been studied mainly for its potential antimineralocorticoid and progestogenic effects, though it is not widely used clinically today.
Oxprenoate potassium is chemically related to canrenoate potassium, a metabolite of spironolactone. Research suggested it might have antiandrogenic properties as well. Due to concerns about tumorigenicity in animal studies with related compounds, some of these progestins were not approved for long-term use.
Chemical Properties
- IUPAC name: Potassium (1S,3aS,3bR,9aR,9bS,11aS)-1-acetyloxy-9a,11a-dimethyl-2-oxo-1,2,3,3a,3b,4,5,6,7,8,9,9a,9b,10,11,11a-hexadecahydrocyclopenta[a]phenanthren-1-yl]acetic acid
- Molecular Formula: C₂₄H₃₃KO₄
- Molar Mass: ~424.61 g/mol
- Appearance: Likely a white or off-white crystalline powder (typical for steroid salts)
- Solubility: Soluble in water (as a potassium salt), more soluble in polar solvents
Pharmacological Properties:
Class: Steroidal progestin and antialdosterone agent
Mechanism of Action:
- Progestogenic activity: Binds to progesterone receptors
- Antimineralocorticoid activity: Competitively inhibits aldosterone at mineralocorticoid receptors, similar to spironolactone
Related Compounds: Spironolactone, canrenone, medroxyprogesterone acetate
Occurrences
Synthetic Origin: It is not naturally occurring; it’s a fully synthetic compound.
Historical/Experimental Use:
- Studied for use in hormonal contraception
- Evaluated as a diuretic or antihypertensive due to aldosterone antagonism
Current Status: Not in widespread clinical use; limited to pharmacological research
Forms: Typically used in salt form (potassium) to improve bioavailability and solubility
Application
Though never widely marketed, it has been investigated for use in treating hormonal disorders, including acne, hirsutism, and conditions influenced by androgens. It works by antagonizing aldosterone receptors, thereby influencing sodium and water balance, and also exhibits weak progestogenic effects. Due to limited clinical use and research, it remains largely of pharmacological interest rather than a mainstream therapeutic agent. Its mechanism resembles that of spironolactone but with distinct pharmacokinetics.