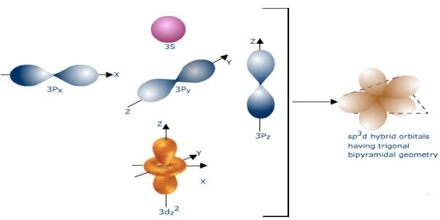
Chemical hybridisation is the idea of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, for example, than the aspect atomic orbitals) ideal for the pairing of electrons in order to create chemical bonds within valence bond principle. Hybrid orbitals are useful in the explanation of molecular geometry and also atomic bonding qualities.
Orbital Hybridisation