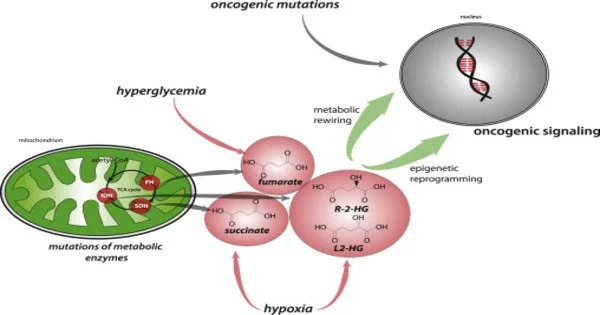
Oncometabolism is the term used to describe the changes in cellular metabolism that occur in cancer cells. It is the study of the metabolic changes that occur in the cells that comprise the tumor microenvironment (TME) and accompany oncogenesis and tumor progression toward a neoplastic state. Normal cells strictly control their metabolism in order to meet the energy and biosynthetic demands of cellular functions.
In terms of metabolism, cells with increased growth and survivability differ from non-tumorigenic cells. The Warburg-Effect describes how cancer cells alter their metabolism to become more oncogenic in order to proliferate and eventually invade other tissues, a process known as metastasis. Cancer cells, on the other hand, frequently exhibit distinct changes in their metabolic pathways to support their rapid growth, proliferation, and survival. These alterations in metabolism are considered one of the hallmarks of cancer.
The chemical reactions associated with oncometabolism are triggered by the alteration of oncogenes, which are cancer-causing genes. During physiological conditions, these genes can be functional and active, producing normal amounts of metabolites. Their upregulation as a result of DNA damage can lead to an excess of these metabolites, which can lead to tumorigenesis. Oncometabolites are metabolites that can function as biomarkers.
Some key features of oncometabolism include:
- Aerobic Glycolysis (Warburg Effect): Cancer cells often exhibit increased glycolysis, even in the presence of oxygen (aerobic glycolysis). This is known as the Warburg effect. In normal cells, the preferred pathway for energy production is oxidative phosphorylation in the mitochondria, but cancer cells favor glycolysis, leading to increased lactate production.
- Altered Mitochondrial Function: Mitochondria play a crucial role in energy production through oxidative phosphorylation. In cancer cells, there are often changes in mitochondrial function, including mutations in mitochondrial DNA, altered mitochondrial dynamics, and changes in the tricarboxylic acid (TCA) cycle.
- Increased Nutrient Uptake: Cancer cells have an increased demand for nutrients such as glucose, amino acids, and lipids to support their rapid growth. Transporters responsible for nutrient uptake are often upregulated in cancer cells.
- Reprogramming of Metabolic Pathways: Oncogenes and tumor suppressor genes have the ability to influence metabolic pathways. The activation of oncogenes such as MYC, for example, can stimulate glycolysis and glutaminolysis, promoting cancer cell growth.
- Deregulated Lipid Metabolism: Cancer cells frequently exhibit lipid metabolism changes, such as increased de novo lipogenesis and changes in fatty acid metabolism.
Understanding oncometabolism is critical for developing targeted cancer therapies. Researchers and clinicians are investigating how to exploit cancer cells’ metabolic vulnerabilities to create drugs that selectively target these aberrant metabolic pathways while sparing normal cells. This strategy is part of the new field of cancer metabolism research.