The chemical compound with the formula NOBr is nitrosyl bromide. It is a red gas with a condensing point that is slightly below room temperature. The formation of nitrosyl bromide from nitric oxide and bromine is of particular interest because it is one of only a few third-order homogeneous gas reactions. Because the reaction is easily and measurably reversible, the rate of decomposition of nitrosyl bromide could be predicted if the standard free energy change as a function of temperature was accurately known.
The reversible reaction of nitric oxide with bromine can result in the formation of nitrosyl bromide. This reaction is noteworthy because it is one of only a few third-order homogeneous gas reactions. At standard pressure and temperature, NOBr is susceptible to photodissociation.
2 NO + Br2 ⇌ 2 NOBr
The reaction rapidly establishes equilibrium when the reactants are mixed.
Part A
At a certain temperature the initial concentration of NO was 0.400 M and that of Br2 was 0.255 M. At equilibrium the concentration of NOBr was found to be 0.250 M. What is the value of Kc at this temperature?
Kc = 21.4
PART B
At this temperature, the rate constant for the reverse reaction is 340. M-2s-1. What is kf for the reaction?
Express your answer to three significant figures and include the appropriate units. Include an asterisk to indicate multiplication in compound units, for example, to write the units for a second-order rate constant type M-1*s-1.
Chemical Equilibrium:
Chemical equilibrium is a reversible state of a chemical reaction system in which the concentrations of reactant and product species do not change significantly over time. In this state, the equilibrium constant “K” is temperature and concentrations at equilibrium dependent. Chemical equilibrium can be thought of like a competition between the system’s forward and reverse reactions.
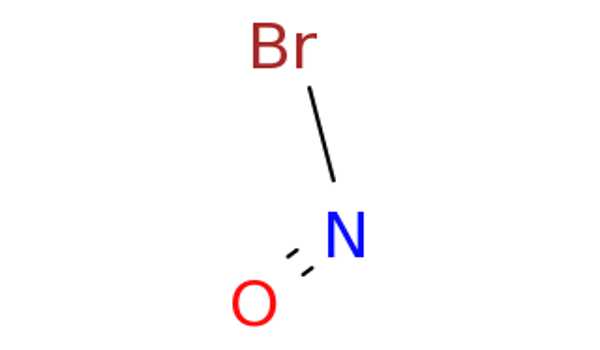
Lewis Structure for NOBr –
- The Lewis structure of NOBr is very similar to that of NOCl and NOF.
- In the NOBr Lewis structure, nitrogen (N) is the least electronegative atom and is located in the Lewis structure’s center.
- Check the formal charges to ensure that each atom has a zero formal charge.
- There are a total of 18 valence electrons in the Lewis structure of NOBr.