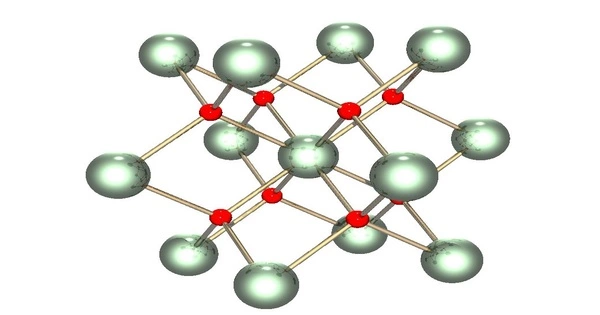
Uranium dioxide (UO2), also known as urania or uranous oxide, is a uranium oxide that occurs naturally as the mineral uraninite as a black, radioactive, crystalline powder. It is a uranium (U) and oxygen (O) chemical combination.
It is one of the most prevalent types of uranium and is used extensively in the nuclear industry, particularly in the manufacture of nuclear fuel for nuclear reactors. It is utilized in nuclear reactors as nuclear fuel rods. MOX fuel is a blend of uranium and plutonium dioxides. Prior to 1960, it was employed in ceramic glazes and glass as a yellow and black color.
Production
Uranium dioxide is produced by reducing uranium trioxide with hydrogen.
UO3 + H2 → UO2 + H2O at 700 °C (973 K)
This reaction plays an important part in the creation of nuclear fuel through nuclear reprocessing and uranium enrichment.
Key properties and facts about uranium dioxide:
- Nuclear Fuel: It is primarily used as fuel in nuclear reactors. When enriched with the fissile isotope uranium-235 (U-235), it can sustain a nuclear chain reaction, releasing a tremendous amount of energy through nuclear fission.
- Ceramic Solid: It is typically a dark black or dark green ceramic solid at room temperature. It has a high melting point and is chemically stable under the conditions found in nuclear reactors.
- Thermal Conductivity: UO2 has excellent thermal conductivity, which is crucial for transferring heat generated during nuclear fission reactions away from the fuel rods to the coolant in the reactor.
- Radioactivity: Natural uranium contains a variety of isotopes, the fissile one being U-235. Because of the existence of uranium isotopes, UO2 used as nuclear fuel is mildly radioactive, and the radioactivity increases as U-235 fissions.
- Nuclear Reactor Fuel Rods: It is frequently formed into cylindrical pellets and stacked to form fuel rods. These fuel rods are inserted within the reactor core and subjected to a controlled nuclear chain reaction to create heat for the generation of power.
- Fission Products: U-235 divides into two or more smaller nuclei during the nuclear fission process in a reactor, producing energy. This process also produces a variety of fission products, including isotopes of other elements, some of which are very radioactive and cause difficulties in nuclear waste disposal.
Applications
Natural uranium is normally enriched to enhance the concentration of U-235 in order to use it as nuclear fuel. This enriched uranium is subsequently turned into uranium dioxide for use in the production of fuel.
High-level radioactive waste is spent fuel assemblies with depleted uranium dioxide. To avoid environmental pollution, these materials must be stored and disposed of properly.
It is critical in the production of nuclear energy, offering a sustainable and efficient source of electricity. However, its use poses serious questions about nuclear safety, waste management, and non-proliferation.