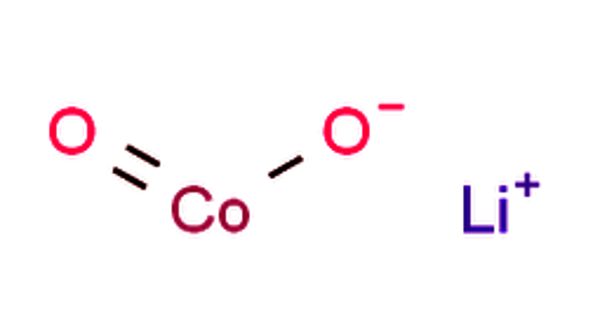
Lithium is the third element in the periodic table is a soft, silvery-white alkali metal. Lithium Cobalt Oxide is a highly insoluble thermally stable source suitable for glass, optic, and ceramic applications. It is sometimes called lithium cobaltate or lithium cobaltite, which is a chemical compound with the formula LiCoO2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide. Currently, the most popular lithium-ion technology is the lithium-cobalt oxide (LCO) battery which has a cathode composed of LiCoO2.
Lithium cobalt oxide is a dark blue or bluish-gray crystalline solid and is commonly used in the positive electrodes of lithium-ion batteries. It is also available in forms such as pellets, pieces, powders, sputtering targets, and nanoparticles
Properties
- Molecular Weight: 97.87
- Appearance: Blue-black, blue, or gray powder
- Melting Point: N/A
- Boiling Point: N/A
- Density: N/A
- Solubility in H2O: N/A
- Exact Mass: 97.939029
Structure
The structure of LiCoO2 has been studied with numerous techniques including x-ray diffraction, electron microscopy, neutron powder diffraction, and EXAFS.
The solid consists of layers of monovalent lithium cations (Li+) that lie between extended anionic sheets of cobalt and oxygen atoms, arranged as edge-sharing octahedra, with two faces parallel to the sheet plane. The cobalt atoms are formally in the trivalent oxidation state (Co3+) and are sandwiched between two layers of oxygen atoms (O2−). The LCO battery has drawbacks in having a low life span, and relatively low safety performance at hot and cold temperatures.
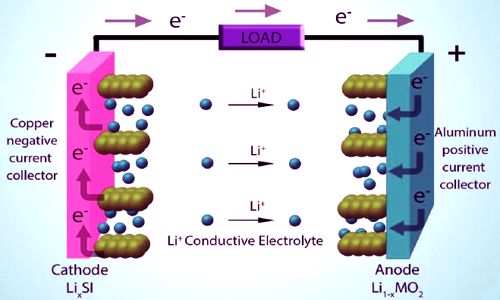
Fig: Lithium Cobalt Oxide Powder for Li-ion Battery
Preparation
Fully reduced lithium cobalt oxide can be prepared by heating a stoichiometric mixture of lithium carbonate Li2CO3 and cobalt (II, III) oxide Co3O4 or metallic cobalt at 600–800°C, then annealing the product at 900°C for many hours, all under an oxygen atmosphere. The technology of LCO is constantly improving with the majority of research focusing on the cathode and electrolyte solution. The anode is considered well optimized allowing for few improvements.
LCO Synthesis
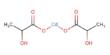
Nanometer-size particles more suitable for cathode use can also be obtained by calcination of hydrated cobalt oxalate β-CoC2O4·2H2O, in the form of rod-like crystals about 8 μm long and 0.4 μm wide, with lithium hydroxide LiOH, up to 750–900°C.
Uses
It has many industrial uses. It goes into glasses, ceramics, pharmaceuticals, and aluminum and magnesium alloys. But the highest potential for growth is in the battery market, where lithium is used as electrode and electrolyte material in lithium disposable batteries and in lithium-ion rechargeable batteries.