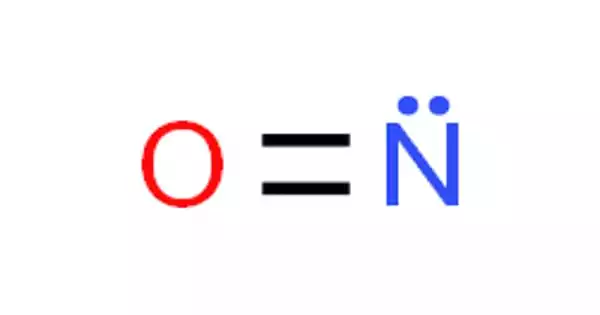
Nitric oxide, sometimes known as NO, is a colorless gas. It is one of the most important nitrogen oxides. It serves key chemical signaling roles in humans and other animals and has a variety of medical applications. Nitric oxide is a free radical, which means that it possesses an unpaired electron, which is commonly represented by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a type of molecule studied in the early modern period that gave rise to theories of chemical bonding. It has a limited number of industrial applications. It is a major air pollutant produced by automobile engines and thermal power plants.
Nitric oxide, an important intermediate in industrial chemistry, occurs in combustion systems and can be produced by lightning in thunderstorms. Nitric oxide is a signaling molecule in numerous physiological and pathological processes in mammals, including humans. In 1992, it was named “Molecule of the Year.” The discovery of nitric oxide’s significance as a cardiovascular signaling molecule earned the 1998 Nobel Prize in Physiology or Medicine.
Properties
Nitric oxide occurs in the form of a colorless gas. Noncombustible, yet hastens the combustion of flammable material. It is free radical nitrogen oxide, with each molecule containing one nitrogen and one oxygen atom. It is extremely hazardous when inhaled and absorbed via the skin. It is a reactive nitrogen species, a nitrogen oxide, an inorganic radical, and an inorganic radical.
Production and preparation
Nitric oxide is created from nitrogen and oxygen through the action of electric sparks or high temperatures, or more simply, through the action of dilute nitric acid on copper or mercury. It was invented in 1620 by Belgian scientist Jan Baptista van Helmont, and it was first studied in 1772 by English chemist Joseph Priestley, who dubbed it “nitrous air.”
In the Ostwald method, nitric oxide is produced commercially by oxidizing ammonia at 750–900 °C (usually 850 °C) with platinum as a catalyst:
4 NH3 + 5 O2 → 4 NO + 6 H2O
The uncatalyzed endothermic reaction of oxygen (O2) and nitrogen (N2), which is effected at high temperature (>2000 °C) by lightning has not been developed into a practical commercial synthesis:
N2 + O2 → 2 NO
Laboratory methods
In the laboratory, nitric oxide is conveniently generated by reduction of dilute nitric acid with copper:
8 HNO3 + 3 Cu → 3 Cu(NO3)2 + 4 H2O + 2 NO
An alternative route involves the reduction of nitrous acid in the form of sodium nitrite or potassium nitrite:
2 NaNO2 + 2 NaI + 2 H2SO4 → I2 + 2 Na2SO4 + 2 H2O + 2 NO
2 NaNO2 + 2 FeSO4 + 3 H2SO4 → Fe2(SO4)3 + 2 NaHSO4 + 2 H2O + 2 NO
3 KNO2 + KNO3 + Cr2O3 → 2 K2CrO4 + 4 NO
The iron(II) sulfate method is straightforward and has been utilized in laboratory investigations at the undergraduate level. NONOate chemicals are also utilized to generate nitric oxide.
Nitric oxide generation is critical for overall health because it permits blood, nutrients, and oxygen to travel properly and efficiently to all parts of the body. In fact, a decreased ability to create nitric oxide has been linked to heart disease, diabetes, and erectile dysfunction.
Applications
- Modern medicine makes use of nitric oxide’s role in controlling blood flow and pressure in a variety of ways.
- It contributes significantly to the air pollution caused by automobile engines and thermal power plants.
- This gas functions as a signaling molecule in the bodies of animals, including humans, and is a critical intermediary in the chemical industry.