Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It has unique electronic properties that make it interesting for various applications, particularly in the fields of electronics and optoelectronics. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride.
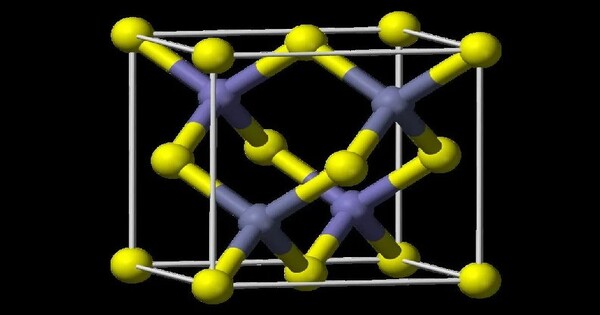
HgTe occurs in nature as the mineral form coloradoite. HgTe has a zinc blende crystal structure and exhibits a narrow bandgap, making it useful for infrared detectors and other applications that require sensitivity to infrared radiation.
Properties
All properties are at standard temperature and pressure unless stated otherwise. The lattice parameter is about 0.646 nm in the cubic crystalline form. The bulk modulus is about 42.1 GPa. The thermal expansion coefficient is about 5.2×10−6/K.
- Chemical formula: HgTe
- Molar mass: 328.19 g/mol
- Appearance: near black cubic crystals
- Density: 8.1 g/cm3
- Melting point: 670°C
Natural Occurrence
Mercury telluride can be found in nature as a mineral, though it is rare. It occurs in some mercury and tellurium deposits.
Synthesis
HgTe is typically synthesized in laboratories through methods such as chemical vapor deposition (CVD) or molecular beam epitaxy (MBE) for research and industrial applications.
Applications
Its applications include infrared sensors, high-speed electronics, and potential use in quantum computing due to its topological properties.
Safety Considerations
Both mercury and tellurium are toxic, so handling and disposal of mercury telluride must be done with care, adhering to safety regulations. Due to its properties, HgTe is used in infrared detectors, high-efficiency solar cells, and other advanced electronic devices. The presence of mercury in HgTe raises environmental concerns, as mercury is toxic and poses risks to health and ecosystems.