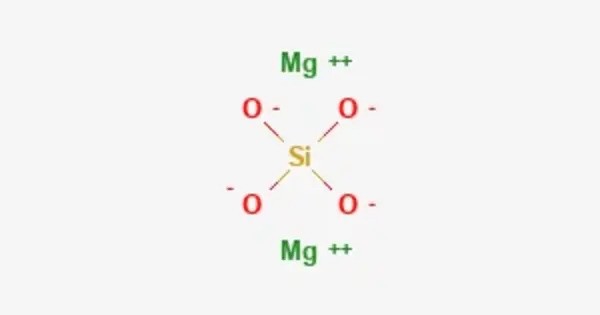
Magnesium orthosilicate is a chemical compound with the formula Mg2SiO4. It is the orthosilicate salt of magnesium. It’s also known by its mineral name forsterite, which is the magnesium-rich end-member of the olivine solid solution series (the other end-member being fayalite, Fe₂SiO₄). It exists as forsterite in nature. It’s a high-magnesium endmember of the olivine solid solution series (the iron-rich endmember being fayalite – Fe₂SiO₄).
Properties
- Chemical formula: Mg2SiO4
- Molar mass: 140.693 g/mol
- Appearance: colorless crystalline solid
- Density: 3.21 g/cm3
- Melting point: 1,890 °C (3,430 °F; 2,160 K)
- Solubility in water: insoluble
Production
Magnesium orthosilicate is made by the fusion of stoichiometric amounts of magnesium and silicon oxides at 1,900 °C (3,450 °F).
Occurrences
- Forsterite is found in igneous and metamorphic rocks, and sometimes in meteorites.
- Igneous Rocks – Common in mafic and ultramafic rocks (e.g., dunite, peridotite). Often an early-forming mineral in basaltic magma.
- Metamorphic Rocks – Forms during contact metamorphism of dolomitic limestones, especially in skarn deposits. Can appear in serpentinite rocks as a residual mineral.
- Meteorites – Occurs in chondrites and other primitive meteorites. Sometimes found as tiny olivine grains in cometary dust and interplanetary particles.
- Gem Quality – Transparent, green varieties are cut as peridot, a gemstone.
Uses
- Geology & petrology: It’s an important rock-forming mineral in Earth’s upper mantle.
- Refractory materials: Due to its high melting point, it’s used in ceramics and refractory linings.
- Gemstone: The gem variety of forsterite is known as peridot.