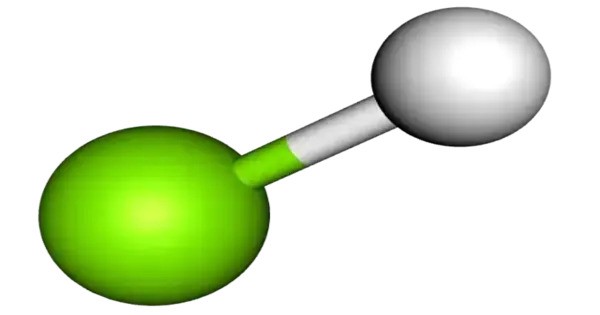
Magnesium monohydride is a binary hydride consisting of one magnesium (Mg) atom and one hydrogen (H) atom. It is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Properties
- Chemical formula: MgH
- Molar mass: 25.313 g/mol
- Appearance: green glowing gas
- Solubility in water: reacts violently
- Bond type: Ionic-covalent character
Formation
- MgH is not commonly found under ambient conditions. It’s typically formed:
- In high-temperature environments like stellar atmospheres.
- Through laser ablation or gas-phase reactions in laboratory settings.
Natural Occurrences
MgH does not naturally occur on Earth in stable, bulk form due to its high reactivity and instability. However, it has been observed in several cosmic contexts:
Astrophysical Environments: Stellar Atmospheres: Detected in the spectra of cool stars (like the Sun and red dwarfs), particularly in G and K-type stars.
Solar Spectrum: MgH molecular bands (e.g., MgH A²Π–X²Σ⁺ transition) are used in studying stellar atmospheres and magnetic field diagnostics.
Brown Dwarfs & Exoplanet Atmospheres: MgH may appear transiently in high-temperature conditions.
Laboratory Synthesis
High-Temperature Reactions: Formed by reacting elemental magnesium with hydrogen gas at elevated temperatures.
Mg (s) + ½ H₂ (g)→MgH (g)
Laser Ablation Techniques: In research, MgH is often produced via laser ablation of Mg in a hydrogen atmosphere and studied in the gas phase using spectroscopy.
Astrophysical Significance
- Detected in stellar atmospheres, especially in cool stars and sunspots.
- Plays a role in determining the chemical composition and temperature of stars.
- Infrared and UV spectroscopy of MgH provides insights into stellar spectra.
Electronic & Spectroscopic Features
- Exhibits rotational and vibrational transitions useful for molecular spectroscopy.
- Has been studied extensively to understand bonding in metal hydrides.
- The bond is polar due to the electronegativity difference between Mg and H.
Stability
- Unlike MgH₂ (magnesium dihydride), MgH is not stable at room temperature.
- Mostly studied in theoretical chemistry, quantum chemistry, and high-energy environments.
Applications
The spectrum of MgH in stars can be used to measure the isotope ratio of magnesium, the temperature, and gravity of the surface of the star. In hot stars MgH will be mostly disassociated due to the heat breaking the molecules, but it can be detected in cooler G, K and M type stars. It can also be detected in starspots or sunspots. The MgH spectrum can be used to study the magnetic field and nature of starspots.
- Astrophysics & Spectroscopy: Plays a key role in stellar atmosphere modeling and solar chemistry.
- Theoretical Chemistry & Quantum Physics: Studied to understand molecular bonding and behavior of metal hydrides.