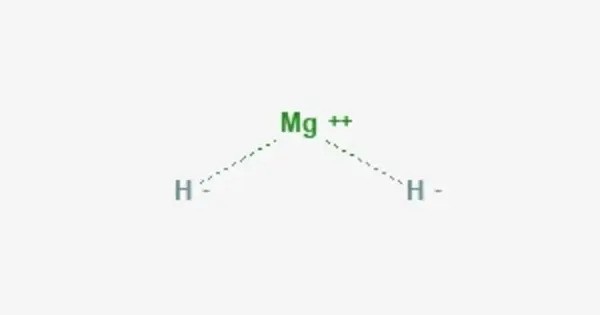
Magnesium hydride is the chemical compound with the molecular formula MgH2. It is a compound of magnesium and hydrogen, primarily known for its role in hydrogen storage and its use in various chemical applications. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Magnesium hydride is an important compound in the field of hydrogen storage due to its ability to absorb and release hydrogen gas. However, its high decomposition temperature and slow kinetics for hydrogen absorption and desorption present challenges. Magnesium hydride does not naturally occur in large quantities and must be synthesized, typically in laboratory and industrial environments.
Properties
- Chemical formula: MgH2
- Molar mass: 26.3209 g/mol
- Appearance: white crystals
- Density: 1.45 g/cm3
- Melting point: 327 °C (621 °F; 600 K) decomposes
- Solubility in water: decomposes
- Solubility: insoluble in ether
- Crystal structure: tetragonal
Preparation
In 1951 preparation from the elements was first reported involving direct hydrogenation of Mg metal at high pressure and temperature (200 atmospheres, 500 °C) with MgI2 catalyst:
Mg + H2 → MgH2
Lower temperature production from Mg and H2 using nanocrystalline Mg produced in ball mills has been investigated.[4] Other preparations include:
the hydrogenation of magnesium anthracene under mild conditions:
Mg(anthracene) + H2 → MgH2
the reaction of diethylmagnesium with lithium aluminium hydride
product of complexed MgH2 e.g. MgH2.THF by the reaction of phenylsilane and dibutyl magnesium in ether or hydrocarbon solvents in the presence of THF or TMEDA as ligand.
Reactions
MgH2 readily reacts with water to form hydrogen gas:
MgH2 + 2 H2O → 2 H2 + Mg(OH)2
At 287 °C it decomposes to produce H2 at 1 bar pressure. The high temperature required is seen as a limitation in the use of MgH2 as a reversible hydrogen storage medium:
MgH2 → Mg + H2
Occurrences
Laboratory and Industrial Synthesis: Magnesium hydride is not typically found in nature in its pure form. It is synthesized in laboratories or industrial settings through reactions involving magnesium with hydrogen gas at elevated temperatures and pressures. A common method of producing MgH₂ is through the reaction of magnesium with hydrogen gas at high temperatures:
Mg + H2 → MgH2
This reaction requires careful control of temperature and pressure.
Occurrence in Magnesium Deposits: While magnesium hydride itself is not naturally abundant in the environment, magnesium is found in large amounts in minerals like magnesite (MgCO₃) and dolomite (CaMg(CO₃)₂). Magnesium hydride is generally produced synthetically rather than occurring naturally.
Hydrogen Storage Systems: Magnesium hydride is of interest in the development of hydrogen storage systems. The high density of hydrogen in MgH₂ makes it a potential material for hydrogen storage applications, though its practical use requires overcoming challenges like high temperature for hydrogen release and the slow kinetics of hydrogen absorption and desorption.
Applications
- Hydrogen Storage: As mentioned, it has potential for use in hydrogen storage systems due to its ability to absorb and release hydrogen.
- Energy Systems: It has been explored in fuel cells and other clean energy systems where hydrogen is needed.
- Metal Hydride Batteries: Magnesium hydride has been studied for use in rechargeable batteries that utilize metal hydrides.