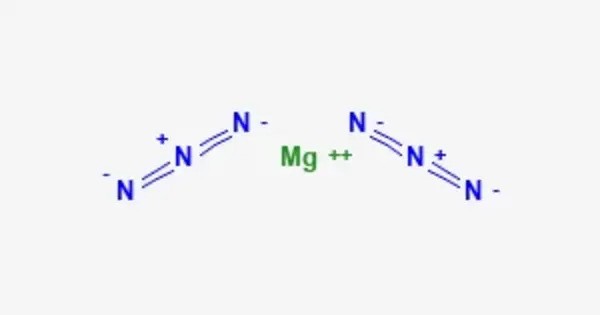
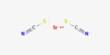
Magnesium azide is an inorganic chemical compound with the formula Mg(N3)2. It is composed of the magnesium cation (Mg2+) and the azide anions (N−3). It’s a potentially explosive compound, due to the reactive nature of the azide group. The azide ion itself is known for its instability and can decompose violently, producing nitrogen gas and sometimes heat or fire. This property makes magnesium azide hazardous to handle.
Magnesium azide can be synthesized by reacting magnesium with sodium azide (NaN₃) in a controlled environment. While its primary use is not common in industry, compounds like magnesium azide have been explored in applications like propellants, explosives, and pyrotechnics, though it’s more likely to be found in specialized research environments rather than in consumer products.
Properties
Magnesium azide hydrolyzes easily. Like most azides, it is explosive. It’s not widely encountered in everyday situations but has applications in fields like explosives and pyrotechnics, similar to other azide compounds.
- Chemical formula: Mg(N3)2
- Molar mass: 108.35 g/mol
- Solubility: It is generally insoluble in water and many organic solvents.
- Stability: It is unstable and should be handled with care, as it can detonate or decompose under certain conditions.
Occurrences
Magnesium azide is usually synthesized in controlled environments, often through reactions involving magnesium compounds and sodium azide (NaN₃). It’s not typically found in nature because the azide ion is highly reactive and not stable under natural conditions.
Use in Explosives and Pyrotechnics
It has applications in explosives, where its decomposition releases large amounts of energy, although it’s not as commonly used as other azides like lead or copper azide.