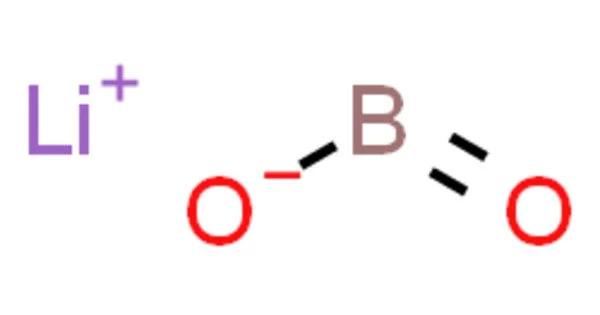
Lithium metaborate, also known as lithium tetraborate or LiBO2, is a chemical compound composed of lithium, boron, and oxygen. It is a white crystalline solid that is soluble in water. It is often encountered as a hydrate, LiBO2·nH2O, where n is usually 2 or 4. However, these formulas do not describe the actual structure of the solids.
Lithium metaborate is one of the borates, a large family of salts (ionic compounds) with anions consisting of boron, oxygen, and hydrogen.
Properties
- Physical properties: It is a white crystalline powder with a melting point of 845°C. It has a density of 2.4 g/cm3 and is soluble in water.
- Chemical properties: It is a stable compound that is not easily decomposed. It is a strong base and reacts with acids to form lithium salts. It is also used as a flux in the analysis of minerals and as a catalyst in chemical reactions.
- Thermal properties: It has a low thermal expansion coefficient and a high thermal conductivity. It is stable at high temperatures and can be used in applications that require heat resistance.
- Optical properties: It has a wide transparent range from ultraviolet to infrared wavelengths. It is commonly used in optical applications such as infrared spectroscopy and crystal growth.
- Electrical properties: It is an ionic conductor and can be used as a solid electrolyte in batteries and fuel cells.
- Mechanical properties: It is a hard and brittle material with a high compressive strength. It can be used as a filler in composite materials to improve their mechanical properties.
Application
Lithium metaborate is used in the preparation of borate fusion beads for X-ray fluorescence analysis, as well as in the production of specialty glasses and ceramics. It is also used as a flux in the metallurgical industry, where it helps to reduce the melting point of metals.
In addition, lithium metaborate is used in the production of lithium-ion batteries, which are used in a wide range of electronic devices such as smartphones, laptops, and electric cars. It is used as a source of lithium ions in the battery electrolyte, which is essential for the battery’s operation.
Toxicity
Lithium metaborate is relatively safe and non-toxic, although it can be irritating to the skin and eyes if contact is made. It is important to handle it with care and use appropriate protective equipment when working with it.
Furthermore, LiBO2 has been investigated for its potential use as a thermal energy storage material due to its high thermal stability and large specific heat capacity. This could make it a promising candidate for use in energy storage applications such as solar power plants.