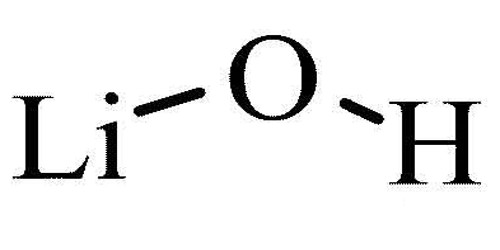Lithium Hydroxide is a chemical compound. Its chemical formula is LiOH. It is a white hygroscopic crystalline material. It contains lithium and hydroxide ions. It used in gas-purification systems in spacecraft, submarines, etc, and in the preparation of lithium soap. It is the weakest base among the alkali metal hydroxides.
Properties
Lithium hydroxide is a white solid. It is totally white in color. While lithium hydroxide is a strong base, it is the weakest known alkali metal hydroxide. It can be anhydrous (without extra water molecules attached) or hydrated (water added to it). It dissolves in water to make a basic solution. It reacts with acids to make lithium salts.
- Chemical formula: LiOH
- Molar mass: 23.95 g/mol (anhydrous); 41.96 g/mol (monohydrate)
- Appearance: Hygroscopic white solid
- Odor: none
- Density: 1.46 g/cm3 (anhydrous); 1.51 g/cm3 (monohydrate); Melting point 462 °C (864 °F; 735 K); Boiling point 924 °C (1,695 °F; 1,197 K) decomposes
- Solubility in water: (anhydrous:) 12.7 g/100 mL (0 °C); 12.8 g/100 mL (20 °C); 17.5 g/100 mL (100 °C;(monohydrate:): 22.3 g/100 mL (10 °C); 26.8 g/100 mL (80 °C)[1]
- Solubility in methanol: 9.76 g/100 g (anhydrous; 20 °C, 48 hours mixing); 13.69 g/100 g (monohydrate; 20 °C, 48 hours mixing)
- Solubility in ethanol: 2.36 g/100 g (anhydrous; 20 °C, 48 hours mixing); 2.18 g/100 g (monohydrate; 20 °C, 48 hours mixing)
Preparation
Lithium hydroxide is made by reacting lithium carbonate with calcium hydroxide.[1] A calcium carbonate solid is made and a lithium hydroxide solution is left behind. It can also be made by reacting lithium with water or by reacting lithium oxide with water.
Uses
It is used in spaceships to absorb carbon dioxide. It reacts with carbon dioxide to make lithium carbonate. This prevents people from suffocating in a spaceship. It is used as a heat transfer medium and as a storage-battery electrolyte. It is also used in ceramics and some Portland cement formulations.
Lithium hydroxide is used to make lithium greases. They are resistant to water and can be used in high or low temperatures. It is used to transfer heat. It can be used in electrolytes. It is also used to prevent corrosion in some nuclear reactors.
Safety
Lithium hydroxide is corrosive, like the other alkali metal hydroxides. It is also a little toxic because it has lithium in it. It may be toxic by ingestion, inhalation and skin absorption. It can burn skin.
Information Source: