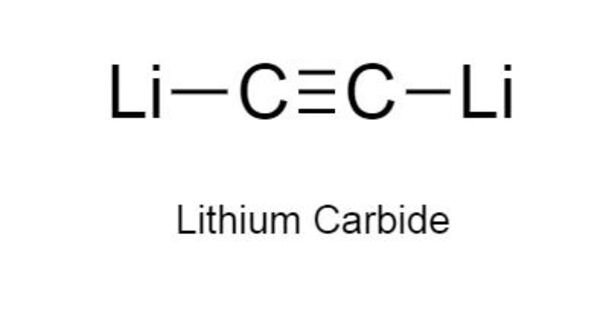Lithium carbide (Li2C2), also known as dilithium acetylide, is a lithium-carbon acetylide molecule. It’s a lithium-carbon compound. It is an ionic compound with an oxidation state of +1 for lithium and -2 for carbon. It is an intermediate chemical formed during radiocarbon dating. Li2C2 is one of many lithium-carbon compounds, including the lithium-rich Li4C, Li6C2, Li8C3, Li6C3, Li4C3, and Li4C5, as well as the graphite intercalation compounds LiC6, LiC12, and LiC18.
Li2C2 is the most thermodynamically stable lithium-rich carbide and the only one that can be produced directly from the elements. Moissan was the first to make it in 1896, by reacting coal with lithium carbonate.
Li2CO3 + 4 C → Li2C2 + 3 CO
The other lithium-rich compounds are produced by reacting lithium vapor with chlorinated hydrocarbons, e.g. CCl4. Lithium carbide is sometimes confused with the drug lithium carbonate, Li2CO3, because of the similarity of its name.
Properties
- Chemical formula: Li2C2
- Molar mass: 37.9034 g/mol
- Appearance: Powder
- Density: 1.3 g/cm3
- Melting point: 452°C
- Solubility in water: Reacts
- Solubility: insoluble in organic solvents
Preparation and chemistry
In the laboratory samples may be prepared by treating acetylene with a solution of lithium in ammonia, on −40°C, with creation of adduct of Li2C2·C2H2·2NH3 that decomposes in stream of hydrogen at room temperature giving white powder of Li2C2.
C2H2 + 2 Li → Li2C2 + H2
Samples prepared in this manner generally are poorly crystalline. Crystalline samples may be prepared by a reaction between molten lithium and graphite at over 1000 °C. Li2C2 can also be prepared by reacting CO2 with molten lithium.
10 Li + 2 CO2 → Li2C2 + 4 Li2O
Other method for production of Li2C2 is heating of metallic lithium in atmosphere of ethylene.
6 Li + C2H4 → Li2C2 + 4 LiH
Lithium carbide hydrolyzes readily to form acetylene:
Li2C2 + 2 H2O → 2 LiOH + C2H2
Lithium hydride reacts with graphite at 400°C forming lithium carbide.
2 LiH + 4 C → Li2C2 + C2H2
Also Li2C2 can be formed when organometallic compound n-butyllithium reacts with acetylene in THF or Et2O used as a solvent, reaction is rapid and highly exothermic.
C2H2 + 2 CH3CH2CH2CH2Li → Li2C2 + 2 CH3CH2CH2CH3
Lithium carbide reacts with acetylene in liquid ammonia rapidly to give a clear solution of lithium hydrogen acetylide.
Li+[−C≡C−]Li+ + HC≡CH → 2 Li+[−C≡CH]
Preparation of the reagent in this way sometimes improves the yield in an ethynylation over that obtained with reagent prepared from lithium and acetylene.