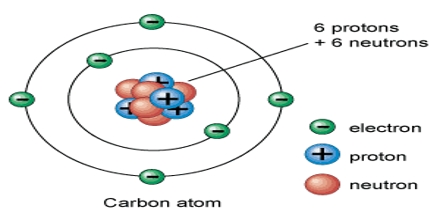
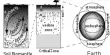
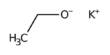
Basic purpose of this lecture is to describe on Atoms. Matter is made up of atoms. The structure of atoms dictate their properties. Protons and neutrons are together in the nucleus of an atom, whereas electrons are in motion in orbits around the central nucleus. Most atoms are electrically neutral, meaning that they have an equal number of protons and electrons. Most atoms are electrically neutral, meaning that they have an equal number of protons and electrons.
Lecture on Atoms