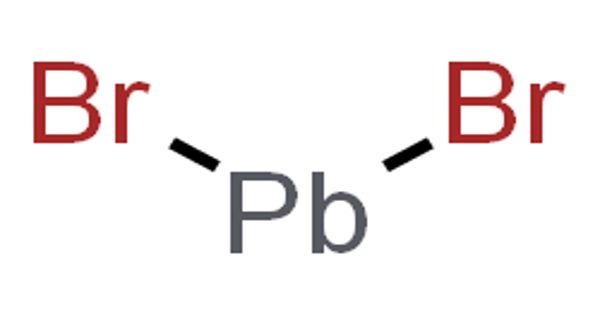
The inorganic compound with the formula PbBr2 is lead(II) bromide. It comes in the form of a white powder. It is produced during the combustion of standard leaded gasoline. It is a white, alcohol-insoluble powder that melts at 373°C and boils at 916°C; it is slightly soluble in hot water.
Preparation
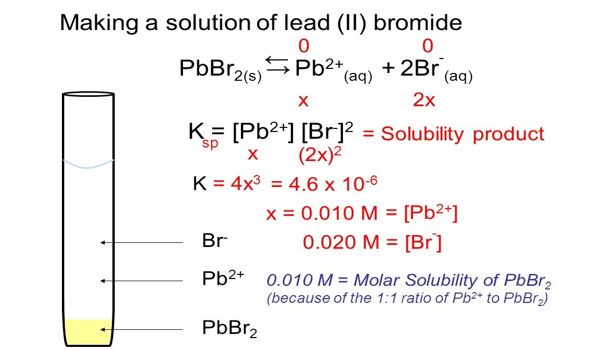
It is typically made by treating lead salt solutions (e.g., lead(II) nitrate) with bromide salts. This method takes advantage of its low water solubility – only 0.455 g dissolves in 100 g of water at 0 °C. In boiling water, it is about ten times more soluble.
PbBr2 and lead chloride (cotunnite) have the same crystal structure – they are isomorphous. Pb2+ is surrounded by nine Br- ions in a distorted tricapped trigonal prismatic geometry in this structure. Seven of the Pb-Br distances are shorter, ranging from 2.9 to 3.3, while two are longer, measuring 3.9. As a result, the coordination is sometimes referred to as (7+2).
The use of electrical energy to split up (decompose) lead bromide is known as electrolysis. The electrical energy is supplied by a d.c. (direct current) battery or power pack. The electrolyte (molten lead bromide), a conducting liquid containing ions, must contain the compound (lead bromide) that is being broken down. To complete the electrical circuit with the battery, electricity must flow through electrodes dipped in an electrolyte.
Properties
As a result of the use of leaded gasoline, lead bromide was prevalent in the environment. Once upon a time, tetraethyl lead was widely used to improve the combustion properties of gasoline. To prevent lead oxides from fouling the engine, gasoline was treated with an organobromine compound that converted lead oxides into the more volatile lead bromide, which was then exhausted from the engine into the environment.
- Molecular Weight: 367.01
- Appearance: White Crystalline Solid
- Melting Point: 373° C (703.4° F)
- Boiling Point: 916° C (1,681° F)
- Density: 6.66 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 367.811265
Application
Lead(II) bromide (PbBr2) can be used to make nanoscale quasi-2D layered perovskites, which could be used to make light-emitting materials. It is also capable of producing deep blue fluorescent lead bromide perovskite microdisks. These microdisks have the potential to be used as direct bandgap semiconductors in light-emitting diodes (LEDs).
Safety
Lead(II) bromide, like other lead compounds, is classified as probably carcinogenic to humans (Category 2A) by the International Agency for Research on Cancer (IARC). Its release into the environment as a byproduct of leaded gasoline sparked intense debate.
Information Source: