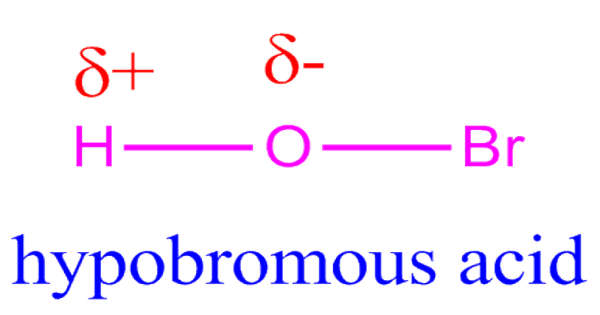
Lead carbide is a hypothetical chemical compound composed of lead and carbon. Even at extreme temperatures, lead and elemental carbon do not usually mix. The modern literature on lead carbide is virtually non-existent. PbC₂ is the chemical formula for this substance. It’s vital to remember that lead carbides, particularly PbC₂, are unstable under normal conditions. Lead often forms more stable compounds with other elements, such as oxides and sulphides, rather than carbides.
Production
In 1923, J. F. Durand reported the synthesis of lead carbide from calcium carbide CaC2 via treatment with an aqueous solution of lead(II) acetate Pb(CH3COO)2, but this discovery was not replicated. A 2007 textbook repeats this assertion, presenting lead carbide as a green powder with the formula PbC2 that is dissolved by hydrochloric acid HCl into acetylene C2H2 and lead(II) chloride PbCl2.
A tiny coating (approximately 10 μm thick) on the inner wall of a graphite crucible used to melt a lead-bismuth eutectic alloy for 100 hours at 1073 K in a helium atmosphere resulted in the discovery of lead carbide PbC2.
In practical terms, lead’s chemistry primarily involves its salts (such as lead acetate and lead chloride) and oxides (like lead oxide). These compounds are more stable and well-documented compared to lead carbides.
Pyrophoric lead
Several accounts of “lead carbide” synthesis occurred in the early nineteenth century, and were widely quoted and copied into textbooks throughout the following decades. In 1820, for example, a certain John claimed to have sublimated a black carbide of lead from a finely divided mixture of lead and charcoal, but this claim appears to have never been repeated. In 1820, Berzelius stated that the pyrolysis (heat decomposition) of iron-lead cyanide produced a double iron and lead carbide, FeC4•2PbC4.
Göbel of Jena discovered a black powder that lit spontaneously when exposed to air in 1823 by pyrolyzing lead tartrate in a closed jar, and he assumed it was a lead carbide. This substance is still widely used in schools to demonstrate pyrophoricity. Shortly thereafter, Proust obtained a similar product from lead acetate and Berzelius obtained one from lead cyanide.
However, by 1870 those pyrophoric residues came to be regarded as an “intimate mixture” of carbon and lead; and the existence of lead carbide was considered unproven.