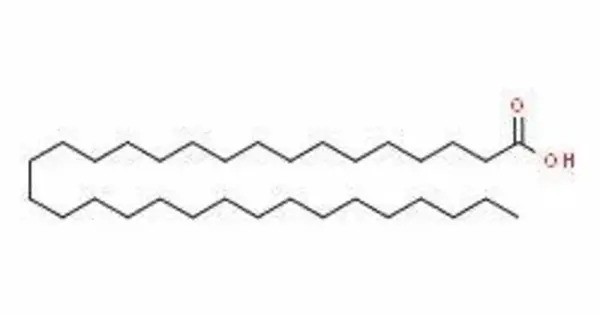
Lacceroic acid (or dotriacontanoic acid) is a saturated fatty acid. It is a lesser-known fatty acid that is primarily associated with certain types of bacteria and marine organisms. typically features a long hydrocarbon chain, characteristic of fatty acids, with specific functional groups that contribute to its unique properties. Like other fatty acids, it is likely to be more soluble in organic solvents than in water, which is a common trait among longer-chain fatty acids.
Sources
Lacceroic acid can be derived by saponification of lacceryl lacceroate or by oxidation of 1-Dotriacontanol (lacceryl) and purification of the product. It can also be isolated from stick lac wax, from which the name is derived.
Properties
- Chemical formula: C32H64O2
- Molar mass: 480.85 g/mol
- Melting and Boiling Points: Specific melting and boiling points would depend on the exact structure, but generally, longer-chain fatty acids have higher melting points.
- Reactivity: It may exhibit reactivity typical of fatty acids, such as undergoing esterification or forming salts.
Occurrences
- Bacterial Sources: Lacceroic acid is often found in the lipid profiles of certain bacteria, particularly those involved in unique ecological niches, such as extreme environments.
- Marine Organisms: It may also be found in marine species, contributing to the complexity of their lipid metabolism.
- Biochemical Role: The role of lacceroic acid in biological systems may involve energy storage or serving as a signaling molecule, akin to other fatty acids.
Research and Applications
Lacceroic acid’s specific applications and functions might still be under investigation, and more studies could shed light on its potential benefits or roles in biotechnology or nutrition.