Isotope electrochemistry is a field within electrochemistry concerned with various topics like electrochemical separation of isotopes, electrochemical estimation of isotopic exchange equilibrium constants, electrochemical kinetic isotope effect, electrochemical isotope sensors, etc.
Chemists are actively working in this area. Electrochemical parametric pumping is a novel separation process in which cycles of reversible electrochemical processes on high surface area electrodes are conducted in synchronization with cycles of solution flow through the separating column. In the present work, isotope separation by electrochemical parametric pumping is studied theoretically. It overlaps with many other chemistry areas of both theoretical and practical importance like nuclear engineering, electrochemical technology, geochemistry, sensors, and instrumentation.
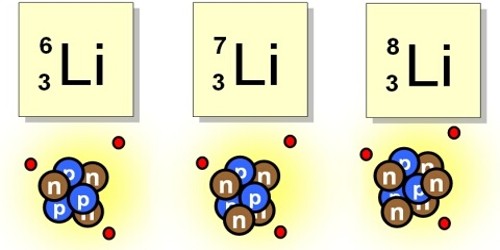
Example: A large electrochemical isotopic effect is observed upon the electrodeposition of lithium from solutions of propylene carbonate producing isotopically light metal deposits. The magnitude of fractionation is controlled by the applied overpotential and is the largest close to equilibrium.
Isotopes of an element contain the same number of protons but different numbers of neutrons. Thousands of tons of isotopic mixtures are processed annually for heavy-water production and tritium decontamination. Isotope Biogeochemistry addresses the application of isotopes of constituents that are dissolved in the water or are carried in the gas phase. Isotopes commonly used in solute isotope biochemistry research include the isotopes of – sulfur, nitrogen, and carbon.