Introduction
Ankylosing Spondylitis (AS) is the major subtype and a main outcome of an inter related group of inflammatory rheumatic disease (spondyloarthropathy) now named spondyloarthritides (SpA) (Braun and Sieper, 2007) showing familial aggregation, arthritis of sacroiliac and peripheral joints with enthesopathy, high association with HLA B27 and absence of rheumatoid factor (Brown et al., 1997; Said-Nahal et al., 2000). Others included in this family are undifferentiated spondyloarthritides (U-SpA), some forms of psoriatic arthritis, reactive arthritis (ReA), and arthritis associated with inflammatory bowel disease (IBD) (Yu et al., 2008). Many of the rheumatic diseases exact etiology and triggering factors are still not known, but a relationship between inflammatory gut lesions and SpA has been demonstrated by many authors (Mielants et al.,1996; Mielants et al., 2005; Praet et al., 2012 )
The prevalence of SpA including AS in inflammatory bowel disease (Crohn’s disease and ulcerative colitis) is high (Mielants and Vey, 2008). Prevalence rates have been described as 10-15% for sacroilitis and of 7-12% for spondylitis, although the figures are probably higher. Some 10% of patients with IBD attending a gastroenterology unit fulfilled the criteria for AS and an additonal 18% of patients had asymptomatic sacroilitis detected by conventional X- ray (Mielants and Veys, 2008).
Although only a small proportion of patients with SpA develop overt IBD, up to two thirds have evidence of subclinical gut inflammation. Ileocolonoscopic studies in patients with SpA have revealed inflammatory gut lesions in 65% of patients with ReA and 57% of patients with AS (Stebbings et al.,2010 ) In studies performed in Belgium and Scandinavia macroscopic and microscopic changes have been identified in patients with SpA in up to 50% (Braun and Sieper, 2007).
Association between HLA-B27 and AS was reported nearly 30years ago .The frequency of HLA-B27 in AS patients ranges from 81 to 96 % while its frequency among the healthy populations is between 4 and 8% (Ebringer et al., 2006).
Gut plays an important role in the pathogenesis of many disorders that fit the concept of the SpA (Narsimulu et al., 2004). A hypothesis has been made that molecular mimicry between enteric bacteria and HLA-B27 plays a pathogenic part in HLA-B27 associated SpA . Researchers have demonstrated cross reactivity between Klebsiella pneumoniae, Yersinia enterocolitica , Shigella flexneri, Shigella sonnie, Salmonella typhimurium, and HLA-B27 (Ringrose, 1999).
The possible interaction between bacteria and HLA B27 has a crucial role in models of pathogenesis of SpA (Braun and Sieper, 2007). Ebringer et al. (2006) postulated that antibody to Klebsiella produced in the gut lymphoid tissues binds to HLA-B27 positive cells especially in entheses of the sacroiliac joints and spinal vertebrae. This causes activation of the complement cascade which leads to cell destruction and inflammation. As the inflammation subsides, a healing process takes place and new bones develops (Schett and Rudwaleit, 2010). Repeated episodes of this ‘Klebsiella reactive arthritis’ eventually produces the clinical syndrome of AS which is due to further bone formation thereby causing fusion of bones (Rashid and Ebringer, 2012).
Associations between inflammatory gut lesions caused by Salmonella, Shigella, Yersinia, Campylobacter and ReA are well established (Keat, 1983; Mielants and Veys, 1984; Clain, 1986). About 10%-20% of HLA B27 positive patients with reactive arthritis develop the full clinical picture of AS after 10-20 years (Braun and Sieper, 2007).
The observation that ReA often followed an enteric infection led to speculation that AS might also be triggered by a gut infection. Serological cross reactivity between HLA-B27 and antigens of Klebsiella pneumoniae has been established. This prompted several groups to study the fecal flora of individuals with SpA (Smith et al., 1997)
The presence and maintenance of these cross-reactive, nonpathogenic bacteria in the bowel flora of patients with AS has been found in a study. This finding suggests that the infectious process in this disorder may be different from other seronegative arthropathies, such as reactive arthritis, in that an acute episode of infection may not occur. The continued isolation of cross-reactive organisms over a period of 12 months would tend to argue for possibility of a subtle, ongoing ‘pathogenic’ process (Predergast et al.,1984)
Of the cross reactive micro-organisms, only Klebsiella could be consistently isolated from fecal cultures obtained from British patients with AS (Ebringer, 1989). In a study of 63 AS patients by Ebringer’s group an increased recovery of Klebsiella could be obtained during the active phase of the disease (Ebringer et al., 1977). A second study showed that AS patients with inactive disease and Klebsiella culture positive subsequently followed by a relapse (Ebringer et al.,1978).
Consideration of the above & other similar findings led Vaughan (1990) to conclude in a recent review ‘The probability that AS is due to a peculiar immunologic relationship between the patient & his enteric organisms & that is determined by his HLA-B27 molecules seems great.
The information on possible source of immune dysregulation in AS is less clear. However enhanced gastrointestinal permeability, the so called leaky gut might be the possible source (Fasano, 2007).
Paneth cells (PCs), the intestinal secretory cells located at the bottom of the intestinal crypts, are well known to play an important role in innate host defence against intestinal micro-organisms. AS patients display a normal number of PC with significant over-expression of PC derived anti- microbial peptides as well as pro-inflammatory cytokines, such as IL-23.This causes pathogenesis of chronic inflammatory gut disorder. Once the disease is manifested, a deficiency in defensin could be the result of loss of epithelial integrity, resulting in bacterial invasion (Cicca et al., 2010).
Both HLA-B27 and gut inflammation play a pivotal role in development of AS. The main etiopathogenetic process is triggered by a combined genetic and environmental (mainly microbial) factors (Rashid and Ebringer, 2011).
The terminal ileum and the large bowel are hospitable places for microbial proliferation because bowel motility is slow here. Lesions of Crohn’s disease usually located where there are microbial residents (ileum and colon). Whereas ulcerative colitis is limited to the large bowel (Harry et al., 2008). The gut microflora (normal flora/microbiota) present in the intestinal lumen differs significantly from the gut flora attached and embedded in the mucosal layer (Sekirov et al., 2010). It has also been reported that the mucosa-associated bacterial communities in the colon are significantly different in composition from those in feces (Zoetendal et al., 2002).
Biopsies unlike feces, provide samples collected from regions of the intestinal tract where inflammation occurs (Rodrigo et al., 2008).Therefore, the study was designed to isolate different aerobic enteric bacteria in colonoscopy biopsy material of patients with AS.
Hypothesis
Enteric aerobic bacteria plays a role in pathogenesis of HLA-B27 positive Ankylosing Spondylitis patients
General Objectives:
To see enteric aerobic bacteria from colonic biopsy material among patients with Ankylosing Spondylitis.
Specific Objectives:
- To isolate enteric aerobic bacteria from colonic biopsy material among patients with Ankylosing Spondylitis and normal healthy controls.
- To observe the difference in bacterial isolates between patients with Ankylosing Spondylitis and normal healthy controls.
- To observe the difference in bacterial pattern between superficial microflora and mucosal bacteria.
- To observe any difference in bacterial load between Ankylosing Spondylitis patients and normal healthy controls.
- To determine the rate of bacterial isolates among HLA-B27 positive patients.
Ankylosing Spondylitis
Ankylosing means fusing together and spondylitis means inflammation of the bones in the spine (from Greek ankylos, stiff; spondylos, vertebrae). AS is a chronic inflammatory disease which was previously known as Bekhterev’s disease, Bekhterev syndrome, or Marie-Strümpell disease, (Braun and Sieper, 2002). It is the major subtype and a main outcome of an inter-related group of rheumatic diseases now named spondyloarthritides showing familial aggregation, arthritis of sacroiliac and peripheral joints with enthesopathy, high association with HLA B27 and absence of rheumatoid factor (Brown et al., 1997; Said-Nahal et al., 2000). Others included in this family are undifferentiated spondyloarthritides (U-SpA), some forms of psoriatic arthritis, reactive arthritis (ReA), and arthritis associated with inflammatory bowel disease (IBD).The subgroups are genetically linked — the strongest known contributing factor is the MHC class I molecule HLA B27 ( Yu et al., 2008).
History
AS has been suggested as the first recognized disease, having been distinguished from rheumatoid arthritis by Galen as early as the second century A.D. However, skeletal evidence of the disease (ossification of joints and entheses primarily of the axial skeleton, known as “bamboo spine”) was first discovered in an archaeological dig that unearthed the skeletal remains of a 5000-year–old Egyptian mummy with evidence of bamboo spine (Braun and Sieper, 2002).
It was not until the late nineteenth century, however, when the neurophysiologist Vladimir Bekhterev of Russia in 1893, Adolph Strümpell of Germany in 1897, and Pierre Marie of France in 1898were the first to give adequate descriptions which permitted an accurate diagnosis of AS prior to severe spinal deformity. For this reason, AS is also known as Bekhterev Disease or Marie–Strümpell Disease (Braun and Sieper, 2002).
Epidemiology
Ankylosing Spondylitis is a disease that affects young people, who generally present at around 26 years of age. Men are more often affected than are women, with a ratio of roughly 2 to 1. About 80% of patients develop the first symptoms at an age younger than 30 years, and less than 5% of patients present at older than 45 years. There is a rough correlation between the prevalence of HLA B27 and the incidence and prevalence of this disease in a specific population. HLA B27 is most prevalent in northern countries and some tribes (with up to 50% of cases), and is highest in Eskimo populations and Haida Indians. Overall, the prevalence of AS is between 0.1% and 1.4%, with most of these data coming from Europe. The incidence of AS is between 0.5 and 14 per 100,000 people per year in studies from different countries (Braun and Sieper, 2007). In a study done in Bangladesh AS was the commonest seronegative disease (28.89% of all inflammatory arthritis) (Hasan et al., 2009).
Pathophysiology
In patients with AS inflammation occurs at the site where certain ligaments or tendons attach to the bone. This area of the body is known as entheses. The inflammation is followed by some erosion (wearing away) of the bone at the site of the attachment. This is known as enthesopathy. As the inflammation subsides, a healing process takes place and new bone develops. Movement becomes restricted where bone replaces the elastic tissue of ligaments or tendons. Repetition of this inflammatory process leads to further bone formation and the individual bones which make up the backbone (vertebrae) fuse together. The pelvis is most commonly affected first. The lower back, chest wall and neck may also become involved at different times (Schett and Rudwaleit, 2010).
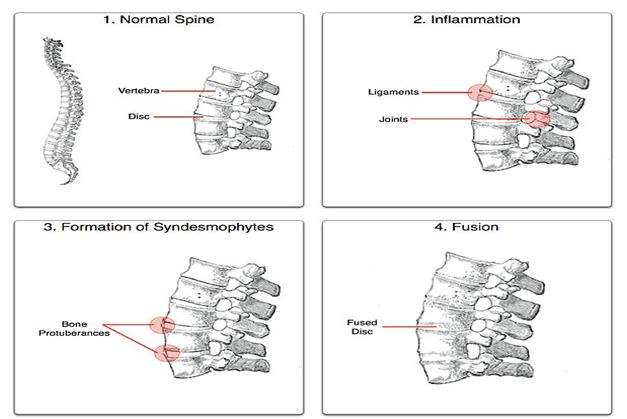
Fig: Ankylosing process
Theories proposed to explain the link with HLA
Human Leukocyte Antigen (HLA) B27 (subtypes B*2701-2759) is a class I surface antigen encoded by the B locus in the major histocompatibility complex (MHC) on chromosome 6 and presents antigenic peptides (derived from self and non-self antigens) to T cells. HLA-B27 is strongly associated with AS, and other associated inflammatory diseases referred to collectively as SpA (Khan, 2010).
The prevalence of HLA-B27 varies markedly in the general population. For example, about 8% of Caucasians, 4% of North Africans, 2-9% of Chinese, and 0.1-0.5% of persons of Japanese descent possess this gene. In northern Scandinavia (Lapland), 24% of people are HLA-B27 positive, while 1.8% have associated Ankylosing Spondylitis.
HLA-B27 consists of a heavy chain having three α domains, which noncovalently binds short peptides and β2-microglobulin. There are 24 HLA-B27 subtypes currently recognized. The structural patterns are consistent with B2705 being the ancestral allele and the other types being generated by small mutations. B2705 is the dominant subtype and is associated with AS across broad ethnic and geographic boundaries. Of the subtypes studied to date, it appears that B2706 and B2709 do not confer susceptibility to AS. Although the HLA-B27 has remained a center of extensive research, the mechanism whereby HLA-B27 confers susceptibility to AS is not well defined.
Several different hypotheses have been proposed.
1) The principle of molecular mimicry is still proposed as a possible mechanism in B27-related pathogenesis. This postulates that the antibodies directed against foreign antigens arising during a bacterial infection are cross-reactive with HLA-B27.
2) In the arthritogenic peptide theory, HLA-B27 binds unique peptides of microbial or self-origin and presents them to CD8+ Tcells . These peptides usually have an anchor arginine residue at their second position and the side chain of arginine is bound in the B pocket of HLA-B27. It was recently reported that CD4+ T cells may be involved in class I–restricted immune recognition.Consequently AS could involve an HLA-B27-restricted CD8+ T-cell or CD4+ T-cell response to microbial or self-peptides.
3) There has been considerable interest in aberrant processing or folding of the heavy chain of HLA-B27. Under normal circumstances cell surface HLA-B27 consists of a heavy chain bound to β2m and peptide. This complex is formed in the endoplasmic reticulum Heavy chain folding of HLA-B27 appears to be slower compared with other HLA alleles, however, possibly because of specific amino acid residues in the B pocket. This misfolded heavy chain is usually removed in endoplasmic reticulum, but in the event of insufficient or unavailable chaperone, peptide, or β2m, misfolded heavy chains are increased. This may increase expression of the protein BiP and generate an unfolded protein response in the endoplasmic reticulum, leading to activation of nuclear factor-Kb (Tsai, 2010).
Ankylosing Spondylitis and gut infection
Although ReA, another B27-related SpA, has a clear relation to antecedent infection, this is less clear for AS. B27-transgenic rats raised in a germ-free environment do not develop inflammatory pathology in the gut or the joints, and induction of arthritis following reintroduction of commensal gut flora supports the notion that such organisms play an important role in the pathogenesis of B27- associated gut and joint inflammation (Rashid and Ebringer, 2011).
Link between HLA-B27 and gut-mediated Arthritic diseases
The association of HLA–B27 with AS is amongst the strongest genetic link with any common disease which has been encountered in the field of rheumatology (Thomas and Brown, 2010). This genetic bond was discovered in early 1970s, where more than 95% of patients with AS have been found to possess HLA-B27 alleles, whilst the frequency of this gene in the general population was below 10% (Brewerton et al., 1973; Schlosstein et al., 1973). Other diseases in the SpA group have lower but different degrees of associations with this allelotype, depending on the clinical presentation and pathological location of the disease (Braun and Sieper, 2010). For example, the frequency of this allelotype in patients with IBD/CD without associated arthritis is comparable to those of the normal population but increases to 40-60% in those patients with spondylitis/sacroiliitis. The frequency of HLA-B27 in patients with ReA/Reiter’s syndrome and U-SpA is ranging between 30 to 90% and 50 to 70%, respectively, meanwhile its frequency in patients with psoriatic arthritis (PsA) with or without peripheral arthritis is around 20%, but it is increased to up to 60% in patients with associated sacroiliitis. From these data results, it appears that a spondyloarthropathic patient presenting with spinal involvement had a higher chance of possessing HLA-B27 genes than those with peripheral joints involvement only (Rashid and Ebringer, 2010).
Gut inflammation and interrelation between different disease
entities of Spondyloarthiritides
Many data support the existence of an inter-connection or inter-relation between different disease entities in the SpA group. For example:
1). The prevalence of AS in patients with UC and CD was found to be reaching 2.6% and 6%, respectively, giving an overall 3.7% prevalence in patients with IBD (Palm et al., 2002). It has also been reported that AS is frequently associated with IBD, with 5-10% of cases having clinical IBD and approximately 70% of cases having subclinical bowel inflammation (Thomas and Brown, 2010), and this link was emphasized previously through an analytical review (Ebringer et al., 2007). Furthermore, HLA-B27 positive patients with IBD were shown to have higher chances of developing AS when compared to those without IBD (Wright, 1978).
2). Many studies have shown macroscopical and/or microscopical features of gut mucosal inflammation, especially in patients with IBD, ReA, AS, and U-SpA (Mielants et al., 2005). In one particular study, gut changes varying from an acute to asymptomatic chronic intestinal inflammation have been observed in about 60% of patients with SpA (Demetter et al., 2002), and more interestingly, those patients who showed signs of articular remissions were preceded by disappearance of the gut inflammation, which supports the concept that the gut is involved in the pathogenesis of SpA.
3). Another fundamental support for the role of gut and the intestinal flora in the development of SpA in relation to the presence of HLA-B27 genes is the absence of arthritis and colitis in germ-free HLA-B27-transgenic rats and the induction of these features when the rats were relocated to non germ-free environment (Hammer et al., 1990).
4). Twenty to 30 percent of patients with unclassified HLA-B27-positive inflammatory rheumatic diseases (Sany et al., 1980) or oligoarthritis have been shown to develop into one form of the definite spondyloarthropathic group such as IBD, ReA, or AS (Schattenkirchner and Kruger, 1987).
5). It has also been stated that more than half of patients with U-SpA will develop AS over a certain period of time (Mau et al., 1988). In a later study, however, no significant differences were observed in some clinico-radiological or genetic features of AS and U-SpA among the Middle Eastern and South Asian populations (Uppal et al., 2006).
It appears from these results that both HLA-B27 and gut inflammation play a pivotal role in the development of SpA, especially AS and CD, and that the main etiopathogenetic process is triggered by a combined genetic and environmental (mainly microbial) factors (Rashid and Ebringer, 2011).
Ankylosing Spondylitis and microbial link
The first evidence of the epidemiological link between microbes and SpA was detected in the early Twentieth Century, where a triad of urethritis, conjunctivitis, and arthritis, being termed as Reiter’s syndrome, was found to follow a dysenteric or venereal infection (Calin, 1998). Reiter’s syndrome was later recognized as a form of ReA and since then each of the triggering bacterial agents including Yersinia, Campylobacter, Shigella, and Salmonella enterogenic bacteria as well as Chlamydia urogenital pathogens has been found to have an approximately equal role in the development of this disease (Leirisalo-Repo, 2005).
Although epidemiological evidence for the involvement of microbial agents in other disease entities of SpA are lacking, a considerable degree of molecular, immunological, as well as microbiological data are available to support the role of Klebsiella pneumoniae in the etiopathogenesis of AS (Rashid and Ebringer, 2011).
Klebsiella and self cross-reactive antigens
Klebsiella microbes possess various antigens which show molecular similarity and immunological cross-reactivity with HLA-B27 or other self-antigens and these have been demonstrated in several independent studies
1) A molecular homology has been found between a hexameric amino acid sequence, “QTDRED” present in both HLA-B*2705 molecules (residues 72-77) and Klebsiella pneumoniae nitrogenase reductase enzymes (residues 188-193) (Schwimmbeck et al., 1989).
2) A structural similarity has been found between the “DRDE” amino acid sequences (residues 596-599) present in the Pul-D secretion protein from Klebsiella pullulanase enzyme, and the “DRED” amino acid motif (residues 74-77) present in the HLA-B27 molecules (Fielder et al., 1995).
3) Another molecular similarity composed of repeated triplet amino acid sequences “G-x-P” has been discovered between Pul-A secretion protein from Klebsiella pullulanase enzyme and collagen types I, III, and IV mainly contained in the ligaments and cartilaginous structures of spinal vertebral large joints and uvea (Fielder et al., 1995).
Fig : Schematic representation of the molecular similarities between Klebsiella and self-antigens
| Klebsiella pneumoniae | ||||||||||||||
| Nitrogenases | Pullulanases | |||||||||||||
| PUL-D | PUL-A | |||||||||||||
| GLN-THR-ASP-ARG-GLU-ASP ASP- ARG- ASP- GLU Gly-x-Pro | ||||||||||||||
| Q | T | D | R | E | D | D | R | D | E | G | x | P | ||
| Q | T | D | R | E | D | D | R | E | D | G | x | P | ||
| GLN-THR-ASP-ARG-GLU-ASP ASP- ARG- ASP -GLU Gly-x-Pro | ||||||||||||||
| HLA-B27 | Collagens I, III, and IV | |||||||||||||
| Self-antigens | ||||||||||||||
( Rashid and Ebringer, 2011)
Evidence for molecular mimicry
1) A molecular homology has been found between a hexameric amino acid sequence, “QTDRED” present in both HLA-B*2705 molecules (residues 72-77) and Klebsiella pneumoniae nitrogenase reductase enzymes (residues 188-193) (Schwimmbeck et al., 1989).
2) A structural similarity has been found between the “DRDE” amino acid sequences (residues 596-599) present in the Pul-D secretion protein from Klebsiella pullulanase enzyme, and the “DRED” amino acid motif (residues 74-77) present in the HLA-B27 molecules (Fielder et al., 1995).
3) Another molecular similarity composed of repeated triplet amino acid sequences “G-x-P” has been discovered between Pul-A secretion protein from Klebsiella pullulanase enzyme and collagen types I, III, and IV mainly contained in the ligaments and cartilaginous structures of spinal vertebral large joints and uvea (Fielder et al., 1995).
Evidence for immunological cross-reactivity
1) Antibodies obtained from a rabbit immunized with HLA-B27-positive lymphocytes showed positive reactions with the antigenic extracts of five gut-inhabited bacterial agents including Klebsiella, Enterobacter, Salmonella, Shigella, and Yersinia microbes indicating the presence of shared cross-reactive antigens (Welsh et al., 1980).
2) Allogeneic anti-HLA-B27 antibodies obtained from human tissue typing sera were found to bind to Klebsiella antigens more than other tissue typing sera (Avakian et al., 1980).
3) Anti-HLA-B27 monoclonal antibodies were found to bind more specifically to 60 and 80 Kd components of Klebsiella (Ogasawara et al., 1986).
4) IgA antibody levels against synthetic peptides carrying Klebsiella or HLA-B27 cross-reactive antigens were found to be elevated in sera of Japanese AS patients compared to rheumatoid arthritis patients or healthy controls, most probably due to the existing bacterial and self cross-reactive antigens in AS patients (Tani et al., 1997).
5) Secretory IgA2 antibodies were found to be significantly increased against type I, III, and IV collagens in sera of Japanese patients with AS when compared to healthy individuals (Tani et al., 1997).
6) Antibodies from sera of AS patients were found to be cytotoxic to HLA-B27-peptide-bearing cells as shown by increased percentage lysis for sheep red blood cells coated with HLA-B*2705 peptide when compared to patients with rheumatoid arthritis and healthy controls, indicating that these autoantibodies probably contribute to the immunological damages which take place at the pathological sites in patients with AS (Wilson et al., 2003).
Molecular mimicry hypothesis and pathogenetic mechanism in
Ankylosing Spondylitis
Rheumatic fever has been recognized for the last five decades as a systemic arthritic disease caused by early Streptococcal upper respiratory infection with resultant pathological lesions affecting collagens in the joints , heart muscles (Towers et al., 2009), and brain tissues causing Sydenham’s chorea (Cardoso, 2011). These lesions are caused by the binding of anti-Streptococcal antibodies to the self cross-reactive antigens at these sites. The same pathogenetic mechanism can be involved in the development of AS where anti-Klebsiella antibodies are binding to the cross-reactive self antigens at the entheses of the large joints, especially spinal vertebrae as the result of complement dependent cytopathic autoantibody reactions with self antigens. It would appear that rheumatic fever, AS, and CD are all autoimmune diseases evoked by antibodies to environmental bacteria, the infectious agents being located at different pathological sites. In patients with AS Klebsiella infection in the bowel, whether overt or subclinical, primarily causes production of anti-Klebsiella antibodies which can also bind to the cross-reactive self antigens like HLA-B27 and collagen fibers in the joints. Recurrent infections with these bacteria could explain the characteristic remission/exacerbation features which have been observed frequently in patients with these diseases (Rashid and Ebringer, 2011) .The entheses in and around the sacroiliac and spinal joints are the principal sites of inflammation in patients with AS (Hamdi et al., 2011). The tendency for the occurrence of pathological lesions at these locations within the territory of the spinal joints is most probably due to their proximity with the draining local lymphatic plexus (Batson’s plexus) from the large bowel in which Klebsiella and other enterobacterial species are abundant (Rashid and Ebringer, 2011).
Other bacteria and Ankylosing Spondylitis
Molecular mimicry to foreign antigens, especially to micro-organisms, possessing antigenic epitopes which are also present on the HLA-B27 molecule, leads to immune recognition of the foreign antigen would lead to an autoimmune, anti-B27 response. Several examples of potential molecular mimicry have been described involving antigens from Klebsiella (Schwimmbeck et al., 1987), Shigella (a putative plasmid-encoded peptide of unknown function) (Stieglitz et al., (1989), Yersinia (the YOP- I plasmid (Tsuchiya et al., 1990), and outer membrane proteins common to many Enterobacteriaceae including one of 19-21 kD (Chen et al., 1987) and the 35-kD (OmpA) antigen (Zhang et al., 1989). An elegant study using engineered hybrid HLA-B27/B7 molecules to map precisely the epitope responsible for one of the crossreactivity between B27 and OmpA is described in this issue (Toubert et al., 1990). In each of these cases antibodies,sometimes monoclonal, cross-react with bacterial antigens and B27. Predictably, the segment of B27 responsible for the crossreactivity turns out to contain the polymorphic residues that distinguish B27 from other HLA antigens (Gaston, 1990).
Proposal for an antimicrobial therapy in patients with Ankylosing
Spondylitis (Ebringer et al., 2006)
Currently the management of patients with AS includes two main approaches:
(A) The first one involves the use of “non-steroidal anti-inflammatory drugs (NSAIDs) and sometimes “disease modifying anti-rheumatic drugs” (DMARDs) or even the use of immunosuppressive and biologic anti-tumour necrosis factor (anti-TNF) therapies.
(B) The second one involves physiotherapy, spa exercises and postural education
to prevent stiffness and deformities.
The NSAIDs and DMARDs and especially the immunosuppressive and biologic agents are effective in alleviating pain, reducing inflammation and improving the quality of life when combined with exercises to maintain physical function. However these drugs cannot reverse the existing spinal lesions and are associated with deleterious side effects, such as an increased risk of serious bacterial infections or even life threatening histoplamosis.
New therapeutic strategies has been proposed:
(1) Antibiotic treatment
Sulphasalazine was and is still considered by many independent groups as one of the most effective and well tolerated drug in the treatment of patients with AS. A 26-week, placebo control trial showed that enteric coated sulphasalazine seemed to be effective and well tolerated in AS patients. In a year placebo controlled trial of sulphasalazine, a reduced frequency of peripheral arthritis was observed in the treated but not in the control group of AS patients. A 6 month randomised, multi centre, double blind, placebo controlled trial of sulphasalzine in AS patients resistant to treatment with NSAIDs showed that sulphasalazine had greater efficiency than placebo. Norwegian AS patients treated with sulphasalazine, showed a significant decrease in total IgA and secretory IgA in the jejunal perfusion fluid when compared to healthy controls. A significant decrease in the concentration of Klebsiella antibodies during the 26 weeks of sulphasalazine treatment has been reported in Finnish AS patients when compared to controls. Sulphasalazine was also found to be beneficial in preventing recurrences and reducing the severity of uveitis associated with AS. Many groups have shown that the anti-microbial
component sulphapyridine is the active moiety of sulphasalazine. Hence the beneficial effects of sulphasalazine could possibly result from the antimicrobial action. AS patients with peripheral arthritis show the beneficial effects of sulphasalazine more readily than those with axial involvement.
Ciprofloxacine was found to be effective in the early treatment of Yersinia reactive arthritis. Yersinia microbes were found to be eliminated from the gut associated lymphoid tissues in 6 of 7 patients receiving ciprofloxacin compared with none of 9 patients receiving placebo. Furthermore patients receiving placebo had higher levels of circulating IgA antibodies against Yersinia than patients treated with ciprofloxacin.
Certain antibiotics have been tested and found to be effective against Klebsiella infections. Some of these antibiotics include: Cephalosporins, aminoglycosides, mezlocillin, piperacillin, ciprofloxacin, aztreonam, trimethoprin-sulfamethoxazole and imipenem. As, yet there is no clinical data to support the beneficial use of antibiotics in AS and a substantial obstacle is the difficulty of identifying early cases when such therapy might abort the disease process.
(2) Low Starch Diet
Dietary starch provides nutrient materials necessary for the growth of gut bacteria. In vitro studies have shown that the mean number of Klebsiella microbes for 3 different sugars (glucose, sucrose and lactose) per gram of substrate was found to be significantly higher when compared to the value obtained following incubation with 11 different amino acids, thus showing that protein components are relatively inefficient substrates for bacterial growth and proliferation. Furthermore, Klebsiella microbes do not seem to grow on plant and fruit cellulose. In a clinical study, bacterial cultures on 47 vegetarian subjects on a high starch/low protein diet were compared to 45 American subjects on an omnivorous diet involving low starch and high protein consumption. The mean number of Klebsiella microbes in the “high starch” group was 30,000 bacteria per gram of feces compared to a value of 700 bacteria per gram of feces in those on a high protein diet.
In another study, it was observed that the majority of AS patients, who were on low starch diet and high in proteins and fruits, claimed a drop in the severity of their symptoms as well as a reduction in their requirements for NSAIDs. This clinical
improvement was found to correlate with a decrease in total serum IgA, which measures gut flora and also a decrease in inflammation as measured by ESR. Thus the use of a “low starch diet” could be helpful in preventing the growth of the gut Klebsiella bacteria, thereby reducing inflammation in these AS patients and producing clinical improvement
It is suggested, therefore, that a combination of Klebsiella specific antibiotics given in
short courses, especially during attacks of “Klebsiella reactive arthritis” together with
a “low starch/high protein-high fruit diet” may have a beneficial effect on AS patients
especially if started during the early stages of the disease. These measures should be in addition to existing methods of treatment, such as NSAIDs and immunosuppressive and biologic agents. Prospective controlled studies are required to determine the relevance of these measures in the treatment of patients suffering from AS.
Type of study:
This is a case control study.
Study period:
The study was conducted during the period July 2011 to June 2012.
Place of study:
This is a collaborating study which was performed between Department of Rheumatology, Department of Microbiology and Immunology, Department of Gastroenterology and Department of Pathology of Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbagh, Dhaka.
Biopsies were collected from Department of Gastroenterology, BSMMU and Comfort hospital, Green road, Dhaka.
Laboratory works were performed in Department of Microbiology and Immunology, BSMMU, Dhaka.
Inclusion criteria:
Ankylosing Spondylitis patients fulfilling the revised Modified New York criterion (1984) were enrolled after getting informed written consent.
Modified New York criteria 1984 for Ankylosing Spondylitis (Braun and Sieper, 2007)
Clinical criteria
• Low back pain and stiffness for longer than 3 months, which improve with exercise,
but are not relieved by rest
• Restriction of motion of the lumbar spine in both the sagittal and frontal planes
• Restriction of chest expansion relative to normal values correlated for age and sex
Radiological criterion
• Sacroiliitis grade ≥2 bilaterally, or grade 3–4 unilaterally
Definite ankylosing spondylitis is present if the radiological criterion is associated with at least one clinical criterion.
Exclusion criteria (Chowdhury et al., 2009)
- A recent history of diarrhea and dysentery (i.e diarrhea in last one month before enrolment)
- History of taking any antibiotics one month before the test.
- Patients taking disease modifying antirheumatic drugs (DMARDs) for more than
Inclusion criteria:
Age and sex-matched patients visiting gastroenterologist for GI symptoms (other than patients with AS and IBD) and was planned for colonoscopy.
Exclusion criteria
- Persons having musculoskeletal problems.
- A recent history of diarrhea or dysentry (i.e diarrhea in last one month before enrolment).
- Persons taking any antibiotics one month before the test.
Sample size:
The sample size determined by following formula
Here,
Zα=1.96 at 95% confidence level (assumed)
Zβ=1.28 at 90% power
p1=0.50 (Anticipated probability of exposure among the case i.e 50% in previous study)
P2=0.26 (Anticipated probability of exposure among the control i.e 26% in previous study)
Sample Size =70. (35 case and 35 control)
Study population:
A total of 35 patients, fulfilling the revised New York criterion (1984) and 20 controls who all underwent colonoscopy biopsy at Department of Gastroenterology, BSMMU and Comfort hospital, Green road, Dhaka between July 2011 to June 2012 were included in the study.
Sampling technique:
A purposive sampling procedure was followed. All the samples were collected within the period of study.
Data collection technique:
Data of cases and controls were recorded in pre designed data sheet.
Type of specimen:
Biopsy specimen from large gut during colonoscopy.
Gut preparation:
Fasting for about 8-10 hours prior to sample collection along with ingestion of tablet laxena to clear the fecal matter from large gut in patients and controls were done (Chowdhury et al., 2009).
Sample collection:
After taking informed written consent from all patients and controls biopsies were taken during colonoscopy by the physician at the Department of Gastroenterology, BSMMU and Comfort hospital, in the following way:
Two biopsies were taken from proximal colon and two from distal colon. The biopsy sites of each were recorded.
One biopsy from proximal and one from distal colon were collected in separate screw- capped bottles containing sterile physiological saline and brought immediately to the Department of Microbiology and Immunology, BSMMU for microbiological study.
One biopsy from proximal and one from distal colon were placed in 10% formalin and sent for histopathological examination in Department of pathology, BSMMU.
Laboratory methods:
A. Sample processing for microbiological study:
Collected biopsies were processed in the Bio-safety Cabinet Level -2 at the Department of Microbiology and Immunology, BSMMU.
Superficial microflora– The average number of bacteria isolated from supernatants of second and fourth wash are also known as normal flora or microbiota (Sekirov et al., 2010).
Mucosal bacteria– Bacteria isolated from debris left after hypotonic lysis represents mucosa associated bacteria (Zoetendal et al., 2002).
Dilution process:
To get 1:10 fold dilution, 100µL supernatant from second and similar amount from fourth biopsy washes from proximal and distal colon were added separately to 900µL sterile normal saline (NS). For 1:10 fold dilution of hypotonic lysis of tissue debris from proximal and distal colon, homogenization was done aseptically in tissue homogenizor. Then 0.1ml of NS was added to it followed by vortexing for 5 minutes. After vortexing 0.9ml of sterile NS was added to it.
B. Bacterial culture:
Aerobic culture was done on samples from both patients and control groups.
The second and fourth biopsy washes and cell debris left after the hypotonic lysis were plated in different selective media after 10 fold dilution.
Inoculation process:
Quantitative culture method was done. 0.1ml of 10 fold diluted supernatant from second and fourth biopsy wash and from cell debris left after hypotonic lysis from proximal and distal colon were plated separately in all selective medias.
Selective medias:
Mac-Conkey agar: Klebsiella pneumoniae, Salmonella spp and Shigella spp.
Salmonella and Shigella agar (S-S agar): Salmonella spp, Shigella spp.
Yersinia SelectiveAgar (YSA): Yersinia enterocolitica.
Brucella agar: Campylobacter spp.
Mac-Conkey agar:
After inoculation in Mac-Conkey agar, culture plates were incubated aerobically at 37ºc for 24 hours.
Salmonella and Shigella agar (S-S agar):
After inoculation in S-S agar culture plates were incubated aerobically at 37ºc for 24 hours.
Brucella agar : After inoculation in Brucella blood agar, culture plates were placed in the candle jar and incubated at 42ºC (microaerophilic condition) for 48 hours (Old, 2008)
Yersinia Specific Agar (YSA):
Direct inoculation without cold enrichment was done in YSA. Culture plates were incubated aerobically at 28ºC for 48 hours (Schiemann, 1979)
Another 0.1ml, 10 fold diluted second and fourth biopsy washes and cell debris left after the hypotonic lysis from proximal and distal colon were added seperately to 0.9ml phosphate buffered saline (pH 7.6) in screw capped vials and refrigerated at 40C for cold enrichment followed by weekly culture in YSA up till 21days (Bockemühl, 2003).
C. Isolation of organism:
If growth was present then colony count and identification of organisms was done from culture plates.
D. Colony count: In 100μl (0.1ml) —————X number of colony present.
In 1000µl (1ml) ——————
As all samples were 10 fold diluted so:
Colony count =
Colony count is expressed in cfu/ml.
Normal bacterial count in large gut is 1011 to 1012 cfu/ml (O’Hara et al., 2006).
E. Identification of organism:
All the organisms were identified by standard microbiological procedure like colony morphology, Gram stain microscopy and relevant biochemical tests (oxidase, catalase KIA, MIU and Simmon’s citrate). (Old, 2008, Duguid, 2008, Coghlan, 2008, Crichton, 2008)
F. HLA-B27:
HLA-B27 identification was done in Rheumatology Department, BSMMU at LABAID hospital, Dhaka.
HLA SSP B27 kit was used for detection of HLA-B27 by the method of Polymerase chain reaction (PCR) in peripheral blood.
HLA-B27 identification was done in 32 patients with AS. In 3 patients with AS HLA-B27 could not be done.
G. Colonoscopy findings or macroscopic findings:
Colonoscopy of cases and controls were done by Gastroenterologist in the Department of Gastroenterology, BSMMU and Comfort hospital, Green road, Dhaka.
Macroscopic lesions were staged as follows (Cuvelier et al., 1987)
Stage0– Normal.
Stage1– Erythema, oedema.friability of mucosa.
Stage2– Ulcerations, linear or aphthoid.
Stage3– Granulation and cobblestoning of the mucosa.
H. Histopathological findings or microscopic findings:
Histopathological examination of colonic biopsy from cases and control were done by Histopathologist in Department of Pathology, BSMMU, Dhaka.
Microscopic lesions were staged as follows (Cuvelier et al., 1987)
Stage0-Normal.
Stage1-Lymphoid hyperplasia, increase in chronic inflammatory cell content in lamina propria with or without eosinophilia but no evidence of cryptitis or epithelial abnormalities /acute inflammation.
Stage2-Diffuse increase of inflammatory cells in lamina propria with partial villous flattening, crypt distortion and reactive hyperplasia of crypt cell epithelium; infiltration of crypt cell epithelium; infiltration of crypt epithelium with neutrophils; crypt abscesses/chronic inflammation.
Stage3– (Aphthous) ulceration with or without epitheloid granulomas / chronic inflammation.
Results
A total of 35 patients with Ankylosing Spondylitis and 20 controls were enrolled in the
study. There were 23 (65.71%) male with a mean age of 28years and 12 (34.28%) female with a mean age of 33 years in patients with Ankylosing Spondylitis. In control group there were 15 (75%) male with a mean age of 22 years and 5 (25%) female with a mean age of 33 years (Table I).
Table II shows bacteria which were isolated from colonic biopsy in patients and controls.
E.coli (only) were isolated from 20 (57.14%) patients and 19 (95%) controls. Klebsiella spp along with E.coli were isolated from 7(20%) patients. Y.enterocolitica with E.coli were isolated from 5 (14.28%) patients. Salmonella spp with E.coli were isolated from 2 (5.7%) patients. Citrobacter spp with E.coli were isolated from one (5%) control. No growth was seen in one (2.85%) patient.
Table I: Distribution of study population
Patients with Ankylosing Spondylitis N=35 | Control n=20 | |||
Number (%) | Avg Age (years) | Number (%) | Avg Age (years) | |
| Male | 23(65.71) | 28 | 15 (75) | 22 |
| Female | 12 (34.28) | 33 | 5 (25) | 33 |
Table II: Isolated bacteria from colonic biopsy in patients and control group
| Isolated bacteria | Patient N=35(%)
| Control n=20(%) |
| E.coli (only) | 20 (57.14)
| 19 (95) |
| Klebsiella spp+ E.coli | 7 (20) | 0 (0) |
| Y.enterocolitica + E.coli | 5 (14.28) | 0 (0) |
| Salmonella spp+ E.coli | 2 (5.71) | 0 (0) |
| Citrobacter spp+ E.coli | 0 (0) | 1 (5) |
| Total number of isolated bacteria | 34(97.14) | 20(100) |
| No growth | 1(2.85)
| 0 (0) |
- Note: E.coli were isolated only or with other bacteria in all 34 cases and 20 controls
Bacterial pattern isolated in superficial microflora and mucosal bacteria is shown in Table III. E.coli (only) were isolated from 20 (58.82%) superficial flora and 20 (58.82%) mucosal bacteria of the same patients. Similarly Klebsiella spp with E.coli were isolated from 7 (20.58%) superficial flora and 7 (20.58%) mucosal bacteria, Y.enterocolitica with E.coli were isolated from 5 (14.70%) superficial flora and 5 (14.70%) mucosal bacteria and Salmonella spp with E.coli were isolated from 2 (5.88%) superficial flora and 2 (5.88%) mucosal bacteria of the same patients. In control E.coli (only) were isolated from 19 (95%) superficial flora and 19 (95%) mucosal bacteria and Citrobacter spp with E.coli were isolated from 1 (5%) superficial flora and 1(5%) mucosal bacteria of the same controls.
Table IV shows isolated bacterial load in patients and control group. Mean colony forming unit of E.coli isolated from superficial flora were 2.1×104cfu/ml, Klebsiella spp were 4.3×103cfu/ml, Salmonella spp were 3.5×103cfu/ml and Y.enterocolitica were 5.8×102 cfu/ml in patients. Mean colony forming unit of E.coli isolated from mucosal bacteria were 3.6×103cfu/ml, Klebsiella spp were 1.6×103cfu/ml, Salmonella spp were 1.7×103cfu/ml and. Y.enterocolitica were 3.0×102 cfu/ml in patients. In control group mean colony forming unit of isolated E.coli were 5.3×103cfu/ml in superficial flora and 9.5×102cfu/ml in mucosal bacteria. Few Citrobacter spp colony forming unit were isolated from superficial flora and mucosal bacteria in one control.
Table III: Comparison between bacterial pattern isolated in superficial microflora
and mucosal bacteria.
| Isolated bacteria | Patient (N=34)
| Control (n=20) | ||
| Superficial flora (%) | Mucosal Bacteria (%) | Superficial flora (%) | Mucosal Bacteria(%) | |
| E.coli (only)
| 20(58.82) | 20(58.82) | 19(95) | 19(95) |
| Klebsiella spp + E.coli
| 7(20.58) | 7(20.58) | 0(0) | 0 (0) |
| Y.enterocolitica+ E.coli
| 5(14.70) | 5(14.70) | 0(0) | 0 (0) |
| Salmonella spp+ E.coli
| 2(5.88) | 2(5.88) | 0 (0) | 0 (0) |
| Citrobacter spp+ E.coli
| 0 (0) | 0 (0) | 1(5) | 1(5) |
- Note: No growth was observed in one patient.
- E.coli were isolated only or with other bacteria in all superficial flora and mucosal bacteria of 34 patients and 20 controls.
Table IV: Comparison between isolated bacterial load in patients and control group
Isolated bacteria | Patient(N=34) | Control(n=20)
| ||
| Superficial flora (cfu/ml)
| Mucosal bacteria (cfu/ml)
| Superficial flora (cfu/ml)
| Mucosal bacteria (cfu/ml)
| |
| E.coli
| 2.1×104 | 3.6×103 | 5.3×103 | 9.5×102 |
| Klebsiella spp
| 4.3×103 | 1.6×103 | 0 | 0 |
| Y.enterocolitica
| 5.8×102 | 3.0×102 | 0 | 0 |
| Salmonella spp
| 3.5×103 | 1.7×103 | 0 | 0 |
- Notes: No growth was observed in one patient.
- E.coli were present only or with other bacteria in all 34 patients and 20 controls.
- Citrobacter spp colony were few in number in one control.
- E.coli and Klebsiella spp count were enumerated on the basis of growth in
Mac Conkey agar.
- Y.enterocolitica count were enumerated on basis of growth in YSA.
- Salmonella spp count were enumerated on the basis of growth in SS agar.
Results of HLA-B27 among the patients with different bacterial isolates is shown in Table V. Thirty five patients enrolled in the study 27 (77.14%) were HLA-B27 positive and 5 (18.51%) were HLA-B27 negative. E.coli (only) were isolated in 20 patients of which 14 (70%) were HLA-B27 positive and 3(15%) were HLA-B27 negative and in 3 patients HLA-B27 was not done. All 7 (100%) patients with isolated Klebsiella spp with E.coli were HLA-B27 positive. Y.enterocolitica with E.coli were isolated in 5 patients in which 4 (80%) were HLA-B27 positive and one (20%) was HLA-B27 negative. Salmonella spp with E.coli were isolated from two patients who all were HLA-B27 positive. HLA-B27 was negative in one patient with no growth of bacteria.
Macroscopic lesions were present in 19 (54.28%) patients and 2 (10%) controls in colonoscopy findings. No macroscopic lesion were seen in 16 (45.71%) patients and 18 (90%) controls. Microscopic lesions were present in 31(88.57%) patients and one (5%) control in histopathological findings. No microscopic lesions were observed in 4 (11.42%) patients and 19 (95%) control (Table VI).
Table V: Results of HLA-B27 among the Ankylosing Spondylitis patients with
different bacterial isolates
|
Different bacterial Isolates | Number of AS patients N=35 | HLA-B27 Status
| |
| HLA-B27 Positive n (%)
| HLA-B27 Negative n (%) | ||
| E.coli (only)
| 17 | 14(82.35) | 3(17.64) |
| Klebsiella spp+ E.coli
| 7 | 7(100) | 0 (0) |
| Y.enterocolitica + E.coli
| 5 | 4(80) | 1(20) |
| Salmonella spp + E.coli
| 2 | 2(100) | 0 (0) |
| No bacterial growth
| 1 | 0 (0) | 1(100) |
| Total
| 32 | 27(84.37) | 5(15.62) |
- HLA-B27 was not tested in three patients with isolated E.coli (only).
- E.coli were present only or with other bacteria in all 34 patients.
Table VI: Macroscopic (colonoscopy) and microscopic (histopathological) lesion in
patients and control group
| Macroscopic lesion
| Microscopic lesion | ||
|
| Present n (%) | Absent n (%) | Present n (%) | Absent n (%) |
Patient(N=35) | 19 (54.28) | 16 (45.71) | 31 (88.57) | 4 (11.42) |
Control(n=20) | 2 (10) | 18 (90) | 1 (5) | 19 (95) |
Comparison between macroscopic lesion seen during colonoscopy and isolated bacteria in patients and controls are shown in Table VII. Macroscopic lesions were present in 5 (14.70%) patients with isolated E.coli (only), 7 (20.58%) patients with isolated Klebsiella spp with E.coli, 5 (14.70%) patients with isolated Y.enterocolitica with E.coli and 2 (5.88%) patients with isolated Salmonella spp with E.coli. No lesions were seen in 15 (44.11%) patients with isolated E.coli (only). In control 17 (85%) patients had no lesions and 2 (10%) patients had lesions in which E.coli (only) were isolated. One Citrobacter spp with E.coli isolated from control had no lesion.
Table VIII shows the result of macroscopic findings in relation to bacteria isolated among the patients with AS. In 20 E.coli (only) isolated from patients 15 (75%) had stage0 with no lesion and 3(15%) had stage1, and 2 (10%) had stage2 lesions. Of the 7 Klebsiella spp with E.coli isolated from patients 3 (42.85%) had stage1 and 4 (57.14%) had stage2 lesions. Patients with isolated Y.enterocolitica with E.coli had 2 (40%) stage1 and 3 (60%) stage2 lesions and those with isolated Salmonella spp with E.coli had 2 (100%) stage2 lesions.
Table VII: Comparison between macroscopic (colonoscopy) findings and isolated
bacteria in patients and control group
Isolated bacteria
| Macroscpopic findings | |||
| Patient (N=34)
| Control (n=20) | |||
| Present n (%) | Absent n (%) | Present n (%) | Absent n (%) | |
| E.coli (only)
| 5(14.70) | 15(44.11) | 2(10) | 17(85) |
| Klebsiella spp + E.coli
| 7(20.58) | 0 (0) | 0 (0) | 0 (0) |
| Y.enterocolitica + E.coli
| 5(14.70) | 0 (0) | 0 (0) | 0(0) |
| Salmonella spp + E.coli
| 2(5.88) | 0 (0) | 0 (0) | 0 (0) |
| Citrobacter spp +E.coli
| 0 (0) | 0 (0) | 0 (0) | 1(5) |
| Total
| 19(55.88) | 15(44.11) | 2(10) | 18(90) |
- Note: No growth was observed in one patient.
- E.coli were present only or with other bacteria in all 34 cases and 20 controls.
Table VIII: Results of macroscopic (colonoscopy) findings in relation to bacteria
isolated among the patients with Ankylosing Spondylitis
Isolated bacteria
|
Number of AS patient | Macroscopic Findings
| |||
| Stage0 n (%)
| Stage1 n (%) | Stage2 n (%) | Stage3 n (%) | ||
| E.coli (only)
| 20 | 15 (75) | 3 (15) | 2 (10 )
| 0 (0) |
| Klebsiella spp + E.coli
| 7 | 0 (0) | 3 (42.85) | 4(57.14) | 0 (0) |
| Y.enterocolitica + E.coli
| 5 | 0 (0) | 4(57.14) | 3 (60) | 0 (0) |
| Salmonella spp + E.coli
| 2 | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Total
| 34 | 15(44.11) | 8(23.52) | 11(32.35) 0 (0) | |
- Note: No growth was observed in one patient.
- E.coli were present only or with other bacteria in all 34 patients.
Comparison between microscopic lesion seen during histopathology and isolated bacteria in patients and controls are shown in Table IX. Microscopic lesions were present in 17 (50%) patients with isolated E.coli (only), 7 (20.58%) patients with isolated Klebsiella spp with E.coli, 5 (14.70%) patients with isolated Y.enterocolitica with E.coli and 2 (5.88%) patients with isolated Salmonella spp with E.coli. No lesion were observed in 3 (8.82%) patients with isolated E.coli (only). In control 18 (90%) patients had no lesion and one (5%) patient had lesion in which E.coli (only) were isolated. No lesion were present in a control with isolated Citrobacter spp with E.coli.
Table X shows the results of histopathological findings in relation to bacteria isolated among the patients with AS. In 20 E.coli (only) isolated from patients 3 (15%) had stage0 with no lesion and 4 (20%) had stage1, 12 (60%) had stage2 and 1 (5%) had stage3 lesions. Of the 7 Klebsiella spp with E.coli isolated in patients 1 (14.28%) had stage1 hand 6 (14.28%) had stage2 lesions. Patients with isolated Y.enterocolitica with E.coli had 3 (60%) stage2 and 2 (40%) stage3 lesions and patients with isolated Salmonella spp with E.coli had 2 (100%) stage2 lesions.
Table IX: Comparison between microscopic (histopathological) findings and
isolated bacteria in patients and control group
Isolated bacteria | Microscopic Findings
| |||
Patient (N=34) | Control(n=20)
| |||
| Present n (%) | Absent n (%) | Present n (%) | Absent n (%) | |
| E.coli (only)
| 17(50) | 3(8.82) | 1(5) | 18(90) |
| Klebsiella spp + E.coli
| 7(20.58) | 0 (0) | 0 (0) | 0 (0) |
| Y.enterocolitica + E.coli
| 5(14.70) | 0 (0) | 0 (0) | 0 (0) |
| Salmonella spp + E.coli
| 2(5.88) | 0 (0) | 0 (0) | 0 (0) |
| Citrobacter spp +E.coli
| 0 (0) | 0 (0) | 0 (0) | 1(5) |
| Total
| 31(91.17) | 3(8.82) | 1(5) | 19(95) |
- Note: No growth was observed in one patient.
- E.coli were present only or with other bacteria in all 34 patients and 20 controls
Table X: Results of microscopic (histopathological) findings in relation to bacteria
isolated among the patients with Ankylosing Spondylitis
Isolated bacteria |
Number of AS patient | Histopathological findings
| |||
| Stage0 n (%)
| Stage1 n (%) | Stage2 n (%) | Stage3 n (%) | ||
| E.coli (only)
| 20 | 3 (15) | 4 (20) | 12 (60) | 1 (5) |
| Klebsiella spp + E.coli
| 7 | 0 (0) | 1 (14.28) | 6 (85.71) | 0 (0) |
| Y.enterocolitica + E.coli
| 5 | 0 (0) | 0 (0) | 3 (60) | 2 (40) |
| Salmonella spp + E.coli
| 2 | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Total
| 34 | 3 (8.82) | 5 (14.70) | 23 (67.64) | 3 (8.82) |
- Notes: No growth was observed in one patient.
- E.coli were isolated only or with other bacteria in all 34 patients
Results of macroscopic, microscopic and bacterial findings among HLA-B27 positive AS patient is shown in Table XI. Of 27 HLA-B27 positive patients 18 (66.66%) had macroscopic lesions, 27 (100%) had microscopic lesions and 18 (66.66%) had both macroscopic and microscopic lesions. HLA-B27 was positive in 14 patients with isolated only E.coli in which 5 (35.71%) had macroscopic lesions 14 (100%) had microscopic lesions and 5 (35.71%) had both macroscopic and microscopic lesion. All 7 patients with isolated Klebsiella spp with E.coli was HLA-B27 positive and also had both macroscopic and microscopic lesion. HLA-B27 was positive in 4 patients with isolated Y.enterocolitica with E.coli and 2 patients with isolated Salmonella spp with E.coli. All of them had macroscopic, microscopic and both lesions. Of the 27 patients with isolated bacteria all were HLA-B27 positive and had no lesion.
Table XI: Results of macroscopic, microscopic and bacterial findings among HLA
B27 positive Ankylosing Spondylitis
Isolated bacteria | HLA- B27 positive AS patients | Macroscopic lesion positive n (%) | Microscopic lesion positive n (%) | Both Macroscopic Microscopic lesion Positive n (%)
| No lesion n (%)
|
| E.coli (only)
| 14 | 5(35.71) | 14(100) | 5(35.71) | 0 (0) |
| Klebsiella spp + E.coli
| 7 | 7(100) | 7(100) | 7(100) | 0 (0) |
| Y.enterocolitica + E.coli
| 4 | 4(100) | 4(100) | 4(100) | 0 (0) |
| Salmonella spp + E.coli
| 2 | 2(100) | 2(100) | 2(100) | 0 (0) |
| Total
| 27 | 18(66.66) | 27(100) | 18(66.66) | 0 (0) |
- Notes: No growth was observed in one patient.
- HLA B27 was not tested in 3 patients with isolated E.coli (only).
- HLA B27 was negative in 3 patients with isolated E.coli (only).
- HLA B27 was negative in one patient with isolated Y.enterocolitica and E.coli.
Discussion
Ankylosing spondylitis (AS) is a chronic inflammatory disease that can cause significant functional complications by affecting the sacroiliac joints and axial skeleton. Despite a longstanding knowledge about the familial associations of this disease, particularly among patients positive for HLA-B27, the fundamental pathogenetic mechanism by which this disease arises in genetically susceptible individuals remains ill defined. Environmental triggers such as infection have not been definitively established but may represent a primary pathogenic step in a molecular-mimicry process (Samji, 2008).
AS patients like reactive arthritis may react in same immunological fashion to some Gram negative bacteria, especially Enterobacteriaceae present in their gut (Trull et al.,
1983). Klebsiella microbes possess various antigens which show molecular similarity and immunological cross-reactivity with HLA-B27 (Rashid, 2011). Antibodies obtained from a rabbit immunized with HLA-B27-positive lymphocytes showed positive reactions with the antigenic extracts of gut-inhabited bacterial agents from Klebsiella, Salmonella, Shigella, and Yersinia (Welsh et al., 1980).The enteric bacteria which shares an outer membrane determinants with the HLA-B27 positive tissue may be involved in early pathogenesis of AS (Prendergast et al.,1984).These findings show that there is a possible role of enteric organisms in the pathogenesis of AS. So, this study was done to isolate enteric aerobic bacteria from large gut of patients with AS and comparing their results with presence and absence of HLA-B27 in them.
The present study includes 35 patients with AS and 20 controls who all underwent colonoscopy. Culture for aerobic enteric bacteria and histopathology were done on all biopsied samples. HLA-B27 tests were done by PCR in patients not in controls.
In the present study average mean age in male was 28years and that of female was 33 years which is similar to the finding of Horst- Bruinsma (2005) where the average age was stated as 28years for both male and female. Majority of patients were male (65.71%) and the male female ratio was 1.9:1. Similar results were seen by Braun et al. (2007) where the ratio was 2:1 and in another study by Will et al. (1990) it was 3:1. The increased prevalence of AS in young adult males may result from a higher starch intake and consequent increase in Klebsiella growth in gut is stated by Anderson et al. (1981). In another study Ebringer et al. (2006) stated that dietary starch provides nutrient materials necessary for the growth of gut bacteria.
E.coli were isolated from all patients and controls but Klebsiella spp, Y.enterocolitica and Salmonella spp were isolated from patients only. Similar results in stool instead of colonoscopy biopsy were reported by Ebringer et al. (1978) in which they isolated Klebsiella from patients with AS. In some studies instead of colonoscopy biopsy they studied serum antibodies against the enteric bacteria and found similar results. Increased level of K. pneumoniae specific antibody in sera were observed in patients with AS by Maki-Ikola et al. (1995). In another study by Tani et al. (1997) it is reported that Japanese patients with AS, have an increased levels of antibodies against Klebsiella, S.enteritidis and S.typhimurium. Wakefield et al. (1989) done a study in Australia, in which out of 15 patients with AS 6 (29%) had positive Y. enterocolitica antibody in the serum. Another study done in United States by Kobayashi et al. (1985), 8 (50%) of 16 patients with AS had antibody against Y.enterocolitica. In this study one colonoscopy sample from patient, showed no growth but the reason of this could not be explained. This may be due to faulty technique.
No difference has been noticed in the bacterial pattern in superficial microflora and mucosal bacteria in patients with AS as well as in control. This finding contradict the finding by Sekirov et al. (2010 ) in which gut microflora present in the intestinal lumen differs significantly from the bacteria attached and embedded in the mucosal layer. Bacteroides, Bifidobacterium, Streptococcus, members of Enterobacteriaceae, Enterococcus, Clostridium, Lactobacillus, and Ruminococcus were all found in superficial flora, whereas only Clostridium, Lactobacillus, and Enterococcus were detected in the mucus layer. The difference in superficial flora and mucosal bacteria were not observed in this study as anaerobic culture was not done.
There is an increase load of E.coli in superficial flora as well as mucosal bacteria in large gut of patients with AS compared to control. Klebsiella spp, Salmonella spp and Y.enterocolitica colony count were not compared as these bacteria were only present in patients. Colony count of gut bacteria in AS patients has not been done previously. Some studies done by other authors on IBD patients showed similar results in which increased bacterial load is associated with gut lesion. In a study by Swidsinki et al. (2002), increased bacterial concentration was observed in IBD patients with chronic bowel inflammation compared to control. In another study Kleesen et al. (2002) observed, increased bacteria in IBD patients in comparison to non IBD patients and stated that pathogenesis of gut inflammation may be due to increased bacterial load. In IBD patients one of some unknown factors, increased bacterial load in the gut stimulates body’s immune system to produce an uncontrolled inflammatory reaction in the intestine. As a result there is inflammation of intestine due to release of inflammatory mediators, such as TNF, IL-12 and IL-23, which causes tissue damage in the intestinal wall (Palmer and Penman, 2010). Similar chronic inflammation in gut has been observed in AS patients (Stebbings et al., 2010). So, increased bacterial load in AS patients may play a role in gut inflammation similarly like in IBD patients.
Among 35 AS patients, 27 (84.37%) were HLA-B27 positive and 5 (15.62%) were HLA-B27 negative and in 3 (8.57%) patients HLA-B27 was not done. Shamji et al. (2008) stated that AS patients has a strong (90%) association with HLA-B27 whereas fewer than 5% of HLA-B27 positive individuals develops AS. This association may be due to high expression of HLA-B27 in peripheral blood mononuclear cells in HLA-B27 positive patients with AS than in healthy HLA-B27 positive individuals (Cauli et al., 2002).The fact that only a small proportion of people positive for HLA-B27 develop AS may be attributable to the different allele types that exist. There are atleast 25 allele subtypes of HLA-B27 that encode 23 different gene products. The subtypes that confer disease susceptibility include B*2705, B*2701, B*2702, B*2704 and B*2707 whereas the B*2706 and B*2709 alleles that are common in Southeast Asian and Sardinia do not have an association with AS (Shamji et al., 2008).
All 7 (100%) patients with isolated Klebsiella spp, 2 (100%) patients with Salmonella spp and 4 (80%) patients with Y.enterocolitica were HLAB-27 positive. Molecular similarities, comprising a hexameric amino acid sequence “QTDRED,” between Klebsiella nitrogenase reductase enzymes and HLA-B27 self-antigen molecules and a quadrimeric homologous structure “DRDE” between Klebsiella pullulanase pul-D molecules and HLA-B27 has been reported by Rashid and Ebringer (2012). Cross reactivity between Klebsiella pneumoniae, Yersinia enterocolitica, Shigella flexneri, Shigella sonnie, Salmonella typhimurium, and HLA-B27 has been reported in another study by Ringrose (1999). The presence of cross reactive enteric bacteria in patients supports the hypothesis that molecular mimicry is a possible mechanism in HLA- B27 related pathogenesis of Ankylosing Spondylitis.
E.coli alone were isolated from 20 AS patients, of which 14 (82.35%) were HLA-B27 positive, 3 (17.64%) were HLA-B27 negative and in 3 patients HLA-B27 was not done. Pathogenesis of AS patients with E.coli can be explained by cross-reactive antigen with Klebsiella which has been demonstrated by Prendergast et al. (1983). In this study all 20 patients with E.coli alone had increased bacterial load and 17 (85%) had gut lesion. Increased load of E.coli has been observed by Kotlowski et al. (2007), Darfeuille–Michaurd et al. (2004) and Conte et al. (2006) in patients with IBD. Carvalho et al. (2009) explained that overgrowth of E.coli can result from host mediated inflammation or abnormal expression of molecules acting as receptors for bacterial adherence. In patients with Crohn’s disease abnormal ileal expression of carcinoembryonic antigen related cell adhesion molecule (CEARCAM) 6, which acts as a receptor for adherent invasive E.coli (AIEC) has been observed. These E.coli adhere to and invade intestinal epithelial cells. They survive and replicate within macrophages leading to the secretion of high amount of TNF leading to gut inflammation. As different strain of E.coli or CEARCAM 6 recepter are not detected in this study, so it cannot be confirmed but similar phenomenon by E. coli could play a role in patients with AS causing chronic gut inflammation.
One (20%) patient with Y.enterocolitica and 3 (17.64%) patients with E.coli were HLA-B27 negative. The reason might be that the pathogenesis of these AS patients was due to some other genes like HLA-B60, HLA-DR1 or HLA-B39 other than HLA-B27. In a study by Kim et al. (2004), it was stated that HLA-B60 and HLA-DR1 has association with AS. In another study by Yamaguchi et al. (1995), 3 (37.5%) patients out of 8 were negative for HLA-B27 but positive for HLA-B39.
Nineteen (55.88%) macroscopic lesions and 31 (88.57%) microscopic lesions were observed in AS patients. Similar study done on Korean patients with AS in which 29–49% macroscopic gut inflammation and 25–62% microscopic inflammatory lesions were observed by Lee et al. (1997). Islam et al. (2010) observed 14.28% macroscopic lesions and 50% microscopic lesions in 28 AS patients. IIleocolonoscopic studies by Mielants et al. (1985) have observed that over 50% of active AS patients have microscopic gut inflammation. Fasano (2007) stated that the gut inflammation may act as leaky gut which enable bacteria or LPS to cross the mucosa. All patients and controls included in the study population had no history of diarrhea. So, in this study AS patients with lesion had silent gut inflammation. Similar findings were seen by Jason et al. (1970) in which 8 AS patients diagnosed with chronic inflammatory gut lesion 3 (37.5%) were symptom free. Three controls in this study had either macroscopic or microscopic lesion which may be due to some other disease as they were patients other than having AS or IBD.
Of 34 patients with isolated bacteria, 19 (55.88%) of them had macroscopic lesions. In these 19 patients, maximum proportion of lesions were stage2 (32.35%), followed by stage1 (23.52%). No stage3 lesions were seen in these patients. Stage0 means no lesion and there were 15 (44.11%) patients with isolated bacteria who had no macroscopic lesions. Of the patients with stage2 lesion meaning presence of ulcer, 72.38% were observed in patients in whom Klebsiella spp with E.coli, Y.enterocolitica with E.coli and Salmonella. Spp with E.coli were isolated. Similar study by Islam et al. (2010) showed 14.28% macroscopic lesion in short colonoscopy (60cm) were isolation of bacteria were not done. In another study by De vos et al. (1989) 30% macroscopic lesion were observed. These findings suggest that increase bowel permeability might play a role in pathogenesis of AS by transferring the crossreactive bacterial antigens to gut lymphoid tissues similarly as been stated by Ebringer et al. (2006). Antibodies produced in the gut lymphoid tissues against these crossreactive bacterial antigens, may predisposes to development of an autoimmune response against HLA-B27 to which they are crossreactive with.
Of 34 patients with isolated bacteria, 31 (91.17%) had microscopic lesions. In these 31 patients maximum (67.64%) lesions were stage2 which indicate chronic inflammation, followed by stage1 (14.70%) and then by stage3 (8.82%) lesions. Similar result in a study by Islam et al. (2010), where 64.28% stage2 and 35.71% stage1 microscopic lesions were observed. Chronic inflammatory gut lesion in patients can be explained by Rashid and Ebringer (2011), who stated that Ankylosing Spondylitis is a chronic disease which takes place due to repeated infection by enteric bacteria in gut having molecular mimicry with HLA-B27. Antibodies produced against these enteric bacteria causes repeated inflammation at entheses of sacroiliac joint or vertebrae due to presence of cross reactive antigen. Repetition of this inflammatory process followed by a healing process of new bone formation, leads to the fusion of vertebrae together causing the clinical symptoms of the disease.
Among all (27) HLA B-27 positive patients, Y.enterocolitica with E.coli were isolated from 4 (14.81%) patients, all 4 (100%) were positive for both macroscopic and microscopic lesion. Similarly Salmonella spp with E.coli were isolated from 2 (7.40%) patients, all were positive for both macroscopic and microscopic lesion. Klebsiella spp with E.coli were isolated from 7 (25.92%) patients of which all 7 (100%) had macroscopic and microscopic lesions. E.coli (only) were isolated from 14 (51.81%) patients of which 5 (35.71%) had macroscopic lesion and all 14 (100%) had microscopic lesions. All patients with HLA-B27 positive having isolated bacteria like Klebsiella spp, Y.enterocolitica, Salmonella spp and E.coli had no history of diarrhea but had lesion in their large gut either macroscopic or microscopic. These findings goes in favor of molecular mimicry hypothesis of pathogenesis of AS stated by Ebringer et al. (2006). In which enteric bacteria especially Kleibsiella who has molecular mimicry with HLA-B27 are able to cross the mucosal barrier due to inflammatory change in gut and presenting their LPS to lymphoid tissue. Antibodies develop against them which then cross react with HLA-B27 present at entheses of sacroiliac joint and spine leading to activation of complement cascade which leads to cell destruction and inflammation.
So, from these above findings it goes with the hypothesis of molecular mimicry that enteric aerobic bacteria like Klebsiella spp, Y.enterocolitica and Salmonella spp play some role in pathogenesis in HLA-B27 positive AS patient having gut inflammation.
Conclusion
- Klebsiella spp, Salmonella spp and majority of Y.enterocolitica spp (80%) were isolated from large gut in HLA-B27 positive Ankylosing Spondylitis patients with gut inflammation in comparison to controls.
- There was no difference between superficial flora and mucosal bacterial pattern in patients with Ankylosing Spondylitis and in controls.
- In patients with Ankylosing Spondylitis increased bacterial load in large gut was observed.
Recommendation
- Large sample size of Klebsiella positive cases of AS would be able to state the significance of this isolated enteric aerobic bacteria in the patients.
- Isolation of bacteria from culture along with detection of these bacterial antibody in serum would be able to correlate with the molecular mimicry hypothesis in patients with AS.
- Anaerobic culture would clear if there is any difference in superficial flora and mucosal bacteria in AS patients.
- As isolation of bacteria from proximal and distal colon were similar, so single biopsy specimen from large gut in patients with Ankylosing Spondylitis should be taken.