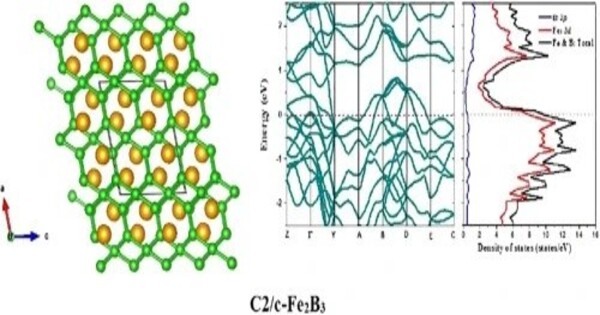
Iron tetraboride (FeB4) is a superhard superconductor (Tc < 3K) consisting of iron and boron. Iron tetraboride does not occur in nature and can be created synthetically. Its molecular structure was predicted using computer models. It is generally a poor conductor of electricity. Its electrical conductivity is significantly lower compared to metals. It can exhibit interesting magnetic properties, including ferromagnetism or antiferromagnetism, depending on its specific crystalline structure and synthesis conditions.
Properties
It has high hardness and is quite brittle. Its hardness makes it useful for various high-wear applications. It is thermally stable at high temperatures, which makes it suitable for high-temperature applications.
- Chemical formula: FeB4
- Molar mass: 99.0920 g/mol
- Crystal structure: orthorhombic
Structure
Iron tetraboride typically has a crystal structure that can be described as a complex boride structure. It’s often found in a tetragonal or orthorhombic lattice configuration.
Applications
Hard Materials: Due to its hardness, iron tetraboride can be used in applications requiring wear-resistant materials. It’s sometimes used as a cutting tool material or in abrasives.
- Magnetic Materials: Its magnetic properties can be utilized in magnetic materials and devices, particularly where specific magnetic characteristics are needed.
- Catalysts: In some cases, iron tetraboride can be used as a catalyst or in catalytic processes, although this is less common.