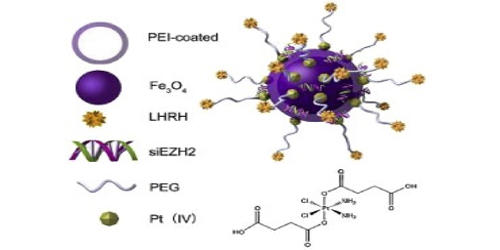
Nanoparticles are of great interest to the scientific community due to their ability to control both quantum and classical coupling interactions. Iron-platinum nanoparticles (FePt NPs) are 3D superlattices composed of an approximately equal atomic ratio of Fe and Pt. They were prepared by the co-reduction of H2PtCl6 and FeCl2 with hydrazine in water/triton X-100/cyclohexane microemulsions. Under standard conditions, FePt NPs exist in the face-centered cubic phase but can change to a chemically ordered face-centered tetragonal phase as a result of thermal annealing. The as-synthesized FePt nanoparticles showed disordered face-centered cubic (fcc) structure. Currently, there are many synthetic methods such as water-in-oil microemulsion, one-step thermal synthesis with metal precursors, and exchanged-coupled assembly for making FePt NPs. The multifunctionality of the developed nanosystem has been realized by catalytic efficiency of platinum for therapeutic application and superparamagnetic property of FePt for magnetic resonance imaging contrast enhancement. The hysteresis loops measured by alternating gradient magnetometer (AGM) revealed that the Pt3Fe exhibited soft magnetic behavior while fct FePt was a hard magnetic material and had higher coercivity.
An important property of FePt NPs is their superparamagnetic character below 10 nanometers. Iron–platinum nanoparticles are predominant amongst these nanostructures. Recently, the cytotoxicity of unmodified iron–platinum nanoparticles was evaluated in endothelial brain cells. Magnetic resonance imaging (MRI), used to track labeled cells, has several advantages, including spatial and temporal resolution and good tissue contrast The superparamagnetism of FePt NPs has made them attractive candidates to be used as MRI/CT scanning agents and high-density recording material.
Properties
Nanoparticles have been attracting considerable attention because of their unique physical and chemical properties in multidisciplinary applications. The various properties of iron-platinum nanoparticles allow them to function in multiple ways. In standard conditions, FePt NPs exist in the face-centered cubic phase with a 3 to 10-nanometer diameter. The crystallite sizes of annealed FePt nanoparticles were 53, 61, and 22 nm for Fe/Pt molar ratios of 3 : 1, 1 : 1, and 1 : 3 respectively. However, once the heat is added the structure becomes a face-centered tetragonal and superparamagnetic. The nanoparticles become superparamagnetic because the addition of heat makes the particle smaller and iron-rich since it removes any impurities in the particles. As a result, the nanoparticles are used in CT or MRI scans.
Plant viruses, known as Cowpea mosaic virus and Tobacco mosaic virus, enlarge the average radius of the FePt NPs through direct mineralization. The physical sizes estimated from transmission electron micrograph (TEM) were similar to those of FePt nanoparticles calculated from X-ray diffraction (XRD) data.