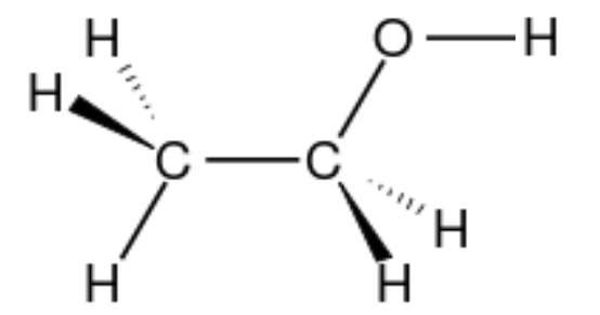
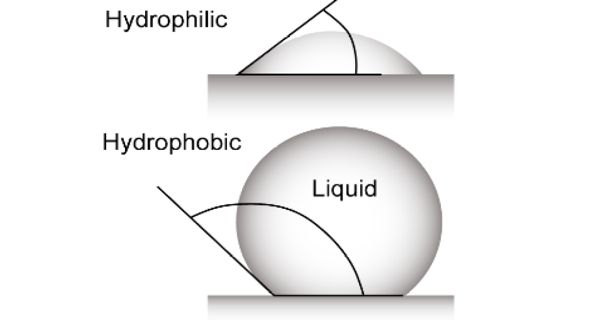
If a molecule is “water-loving”, it is known as ‘hydrophile’ (noun) that possesses a hydrophilic nature. A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water. It means “water-loving”. If a molecule has areas where there is a partial positive or negative charge, it is called polar, or hydrophilic. Polar molecules dissolve easily in water.
In contrast, hydrophobes are not attracted to water and may seem to be repelled by it. The opposite of hydrophilic is hydrophobic, or water-hating. Hygroscopic are attracted to water but are not dissolved by water. When hydrophilic materials combine with water, a thermodynamic interaction is more favorable than the interactions with oil or other hydrophobes.
Molecules
Hydrophilic substances are polar in nature. A hydrophilic molecule or portion of a molecule is one whose interactions with water and other polar substances are more thermodynamically favorable than their interactions with oil or other hydrophobic solvents. Starch is an example of a hydrophilic polymer. They are typically charge-polarized and capable of hydrogen bonding. Cellulose fibers are hydrophilic due to the presence of -OH groups at their surfaces. This makes these molecules soluble not only in water but also in other polar solvents. ‘Like dissolves like’ theory governs the fact that hydrophilic substances tend to readily dissolve in water or polar solvents while hydrophobic substances are poorly soluble in water or polar solvents.
Hydrophilic molecules (and portions of molecules) can be contrasted with hydrophobic molecules (and portions of molecules). Hydrophilic membranes are basically nonporous films that breathe via ‘osmotic potential’ by adsorbing and desorbing the vapor molecules and then conveying them to the other side, much like cells in the human body allow diffusion of monomolecular and ionic species through their membrane walls. In some cases, both hydrophilic and hydrophobic properties occur in a single molecule. An example of these amphiphilic molecules is the lipids that comprise the cell membrane. Another example is soap, which has a hydrophilic head and a hydrophobic tail, allowing it to dissolve in both water and oil.
Hydrophilic contaminants are common in the environment and could be either organic or inorganic in nature. Hydrophilic and hydrophobic molecules are also known as polar molecules and nonpolar molecules, respectively. Some hydrophilic substances do not dissolve. This type of mixture is called a colloid.
Information Source: