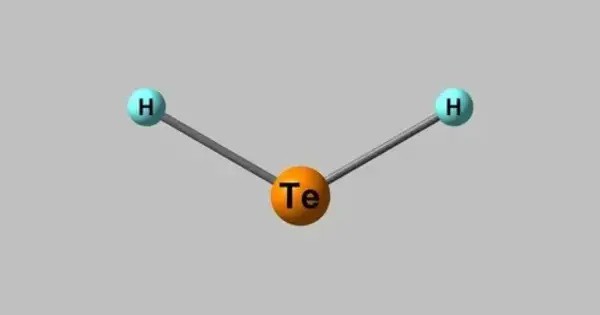
Hydride tellurides are chemical compounds composed of hydrogen and tellurium. These are mixed anion compounds containing both hydride and telluride ions. They are in the category of heteroanionic chalcogenides, or mixed anion compounds. The most fundamental member is hydrogen telluride (H₂Te), a hydride of tellurium analogous to hydrogen sulfide (H₂S) and hydrogen selenide (H₂Se). H₂Te is a colorless, highly toxic gas with an unpleasant odor, unstable under normal conditions. It decomposes readily to elemental tellurium and hydrogen, especially when exposed to heat or light.
Structurally, H₂Te adopts a bent molecular geometry similar to water, with a bond angle around 90°. It is a weak acid in aqueous solution, ionizing to form telluride ions (Te²⁻). These ions can react with metals to form metal tellurides, which are important in semiconductors and thermoelectric materials.
Hydride tellurides exhibit increasing instability and reducing power compared with lighter chalcogen hydrides due to weaker Te–H bonds. Their chemical behavior is dominated by high reactivity and tendency to oxidize, forming tellurium oxides or elemental tellurium.
Formation
Salt-like hydride tellurides may be formed by heating tellurium with a metal hydride in an oxygen-free capsule at around 700°C with a caesium chloride flux to assist in crystal formation. For rare earth elements, this method works as long as tellurium has enough oxidising power to convert a +2 oxidation state to a +3 state. So for samarium, europium, thulium and ytterbium it does not work as the monotelluride is more stable with the metal in a +2 oxidation state. Also scandium and lutetium atoms are too small.
LaH3 + Te → LaHTe + H2
Tellurido-hydrido-transition metal complexes are known. They are formed by heating metal hydrido complexes with tellurium.
L2MH3 + 2 Te → L2M(Te2)H + H2
L2MH3 + Te → L2M(=Te)H + H2
where L and M represent.
In nature, hydride tellurides are rare, as H₂Te is unstable and rapidly decomposes. They are mainly studied under controlled laboratory conditions. Though limited in direct applications due to toxicity and instability, hydride tellurides are significant in theoretical chemis
Properties
With rare earth elements the hydride tellurides all have the same structure as the small or heavy rare-earth elements. Six selenium ions are arranged in a trigonal prism around the rare-earth atom. The rectangular faces are capped with hydride ions. Bond lengths are about the same as for binary tellurides and hydrides.
When heated to 1100°C, rare-earth hydride tellurides decompose to a monotelluride:
2LaHTe → 2LaTe + H2
- Physical state: H₂Te is a colorless gas at room temperature but condenses to a liquid at low temperatures.
- Odor: It has a foul, unpleasant smell, similar to rotten garlic.
- Polarity: Due to its bent molecular geometry, it is a polar molecule, though less so than water.
- Acidity: It is a weak acid in aqueous solution, stronger than H₂S and H₂Se but weaker than H₂O.
- Toxicity: Highly toxic and unstable; it decomposes easily into elemental tellurium and hydrogen.
- Thermal stability: Less stable than other chalcogen hydrides, decomposing upon heating.
Occurrences
Hydride tellurides are extremely rare in nature due to their instability. They do not occur in free form in the Earth’s crust. Instead, they are typically generated in controlled laboratory conditions through:
- Direct reaction of tellurium with acidic hydrides.
- Reduction of tellurium compounds in acidic environments.
Because of their instability and toxicity, hydride tellurides have no significant natural occurrence or widespread industrial use, but they are studied in inorganic chemistry for understanding chalcogen hydride trends and potential semiconductor applications.