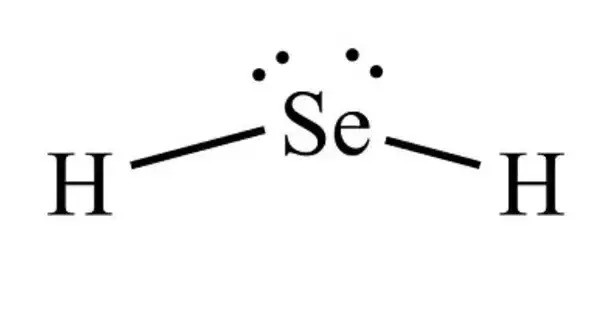
Hydride selenides are chemical compounds that contain hydrogen and selenium, often represented as H₂Se or as more complex hydrogen–selenium species. These are mixed anion compounds containing both hydride and selenide ions. The simplest member is hydrogen selenide (H₂Se), a colorless, toxic, and highly flammable gas with a sharp, irritating odor. Structurally, H₂Se is analogous to hydrogen sulfide (H₂S), consisting of a bent molecule with selenium at the center bonded to two hydrogen atoms. It is heavier than air and soluble in water, where it acts as a weak acid, producing hydroselenide ions (HSe⁻).
Hydride selenides are generally unstable and can decompose upon heating or in the presence of light. H₂Se is highly reactive, easily oxidizing to elemental selenium or reacting with metal cations to form metal selenides, which are important in semiconductor and photovoltaic applications. Due to its high toxicity and low detection threshold, exposure to hydrogen selenide is dangerous, causing respiratory damage and systemic poisoning even at low concentrations.
In nature, selenium hydrides are rare, though traces of H₂Se may form during anaerobic microbial processes involving selenium compounds. Industrially, H₂Se is produced by treating metal selenides with acids. Despite its hazardous nature, it plays a role in chemical vapor deposition and materials research.
Formation
Salt-like hydride selenides may be formed by heating selenium with a metal hydride in an oxygen-free capsule. For rare earth elements, this method works as long as selenium has enough oxidising power to convert a +2 oxidation state to a +3 state. So for europium and ytterbium it does not work as the monoselenide is more stable.
One transition metal complex was formed from a lithium zirconium hydride complex in solution reacting with diphenylphosphine selenide.
Properties
With rare earth elements there are two structure depending on the size of the metal ions. The large atoms form a 2H hexagonal anti-nickel arsenide structure, with hydrogen inserted into tetrahedral positions. A 1H hexagonal structure is found in rare earth elements from gadolinium to lutetium, and yttrium.
- Physical state: H₂Se is a colorless gas at room temperature with a characteristic foul odor, similar to rotten horseradish or decayed radish.
- Toxicity: Extremely poisonous and more toxic than hydrogen sulfide. Exposure to even trace amounts can cause respiratory and nervous system damage.
- Solubility: Moderately soluble in water, forming hydroselenic acid (H₂Se(aq)), a weak acid compared to H₂S but stronger than H₂Te.
- Stability: Thermally unstable, decomposing slowly at room temperature and more rapidly upon heating to elemental selenium and hydrogen.
- Chemical behavior: Acts as a weak reducing agent. It reacts with metals to form metal selenides.
Occurrences
H₂Se does not occur freely in nature due to its instability and high reactivity. It can be formed by the reaction of water or acids with metal selenides (e.g., FeSe, ZnSe).
Trace amounts may occur in the anaerobic decomposition of selenium-containing organic matter, especially in sediments where microbes reduce selenium compounds. It is also detected in industrial processes involving selenium refining, metallurgy, and semiconductor manufacturing.