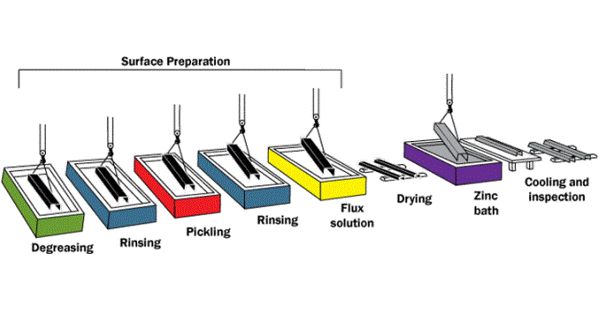
Hot-dip galvanizing (HDG) is the process of coating fabricated steel by immersing it in a bath of molten zinc. It is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around 449°C (840°F). It provides a number of benefits to the steel it protects.
Hot-dip galvanization is the process developed to prevent steel from corroding. It is the most common procedure for coating steel with zinc.
When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2) to form zinc carbonate (ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances. The metallurgically-bonded zinc-iron alloy layers not only create a barrier between the steel and the environment but also cathodically protect the steel. Due to the fact that hot-dip galvanizing has been used to protect iron/steel for such a long time, there are a variety of terms that have been used to describe the process, including galvanization, galvanizing, and hot-dip galvanization.
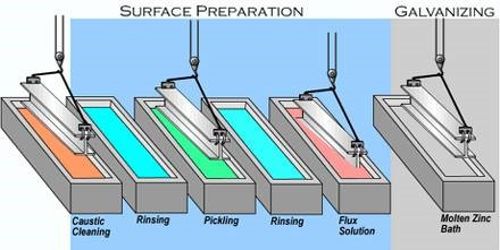
When clean steel is immersed into molten zinc, a series of zinc-iron alloy layers are formed by a metallurgical reaction between the iron and zinc, providing a robust coating that is an integral part of the steel. Galvanized steel is widely used in applications where corrosion resistance is needed without the cost of stainless steel and is considered superior in terms of cost and life-cycle. It offers coverage both externally and internally within hollow sections, it self-repairs when damaged, sacrifices itself to protect the base metal, is environmentally sustainable, has a good impact and abrasion-resistance, and maintenance-free a life of 50 years or more.
Galvanized steel can be welded; however, one must exercise caution around the resulting toxic zinc fumes. The cathodic protection offered by zinc means the galvanized coating sacrifices itself to protect the underlying base steel from corrosion. Galvanized fumes are released when the galvanized metal reaches a certain temperature. This temperature varies by the galvanization process used. Galvanized steel is widely used in applications where corrosion protection is needed and can be identified by the crystallized pattern on the surface.
Electrogalvanized sheet steel is often used in automotive manufacturing to enhance the corrosion performance of exterior body panels; this is, however, a completely different process that tends to achieve lower coating thicknesses of zinc. This may be a batch process known as general galvanizing or a continuous coating of coils of steel strip.
Information Source: