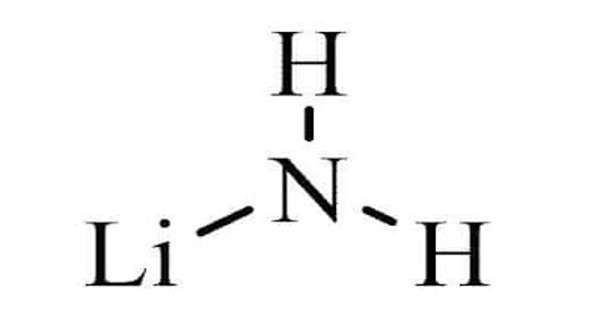
Hexavalent chromium [Cr(VI)] is one of the valence states (+6) of the element chromium. It is a form of the metallic element
chromium. It is the chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the salt sodium dichromate. It is usually produced by an industrial process. Approximately 136,000 tonnes (300,000,000 lb) of hexavalent chromium were produced in 1985.
Chromium is a naturally occurring element found in rocks, animals, plants, soil, and volcanic dust and gases. Additional hexavalent chromium compounds are chromium trioxide and various salts of chromate and dichromate, among others. The Cr(VI) compound chromic acid is used to electroplate chromium onto metal parts to provide a decorative or protective coating. Hexavalent chromium is used in textile dyes, wood preservation, anti-corrosion products, chromate conversion coatings, and a variety of niche uses. Industry adds chromium (Cr) to iron and nickel to make metal alloys especially characterized by their high resistance to corrosion and oxidation.
Hexavalent chromium is a toxic, heavy metal commonly used as an oxidizing agent for stainless steel production, welding, chrome pigment production, and chromium plating. Hexavalent chromium exposure occurs through breathing it in, ingesting it in food or water, or direct contact with the skin. It is hazardous when breathed in, ingested, or touched. Environmental exposure to chromium likely impacts millions of people drinking Cr contaminated water and residing near toxic sites and chemical manufacturers. It has serious health and safety problems and is closely regulated, hence a number of chromate-free processes have been developed.
Uses
Hexavalent Chromium is widely used in electroplating, stainless steel production, leather tanning, textile manufacturing, and wood preservation. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anticorrosive agents to paints, primers, and other surface coatings; and chromic acid electroplated onto metal parts to provide a decorative or protective coating. Hexavalent chromium can be formed when performing “hot work” such as welding on stainless steel or melting chromium metal. It’s used in electroplating, welding, and chromate paint. It can also be found in drinking water and public water systems.