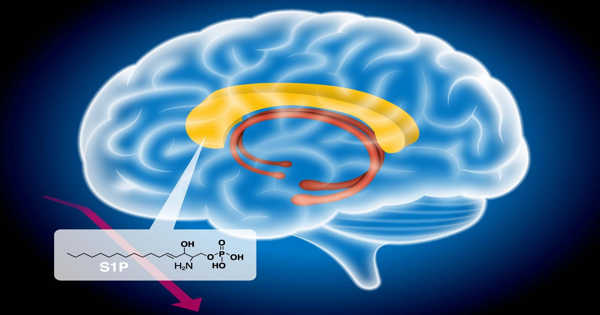
A research team at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) has successfully used magnetotactic bacteria, a unique type of bacteria, to cleanse water that contains uranium. The name derives from their ability to react to magnetic fields.
In their cell walls, they are able to accumulate dissolved heavy metal. The interaction between uranium and bioligands is now well understood as a result of these research findings.
“Our experiments are geared towards potential industrial applications in the field of microbiological remediation of water, especially when it is contaminated with heavy metals of the type you find in mine drainage water in the old uranium mines,” explains Dr. Evelyn Krawczyk-Bärsch of HZDR’s Institute of Resource Ecology.
“For this project we sought help from a very special group of living creatures: the magnetotactic bacteria,” her colleague, Dr. Johannes Raff, adds and continues, “Due to their structure, they are positively predestined for such a task.”
Magnetotactic bacteria create nanoscopic magnetic crystals inside of the cell as a characteristic that sets them apart from other bacteria. They are so neatly shaped and set in a row like beads that humans are currently unable to create them synthetically. Each individual magnetic crystal is embedded in a protective membrane.
The magnetosome is a structure made up of membrane and crystals that the bacteria utilize to orient themselves in their environment and align with the Earth’s magnetic field. It also makes them suitable for simple separation processes.
Nearly every aquatic habitat, including those with very low nutrients and salinity, can support magnetotactic bacteria. Microbiologist Dr. Christopher Lefèvre has even discovered them in the hot springs of Nevada. It was from him and his colleague Dr. Damien Faivre of the French Alternative Energies and Atomic Energy Commission (CEA) that the Rossendorf scientists acquired their bacteria strain, not to mention expert advice on how best to preserve them because despite being fairly common, cultivating them requires some specialist knowledge.
Our experiments are geared towards potential industrial applications in the field of microbiological remediation of water, especially when it is contaminated with heavy metals of the type you find in mine drainage water in the old uranium mines.
Dr. Evelyn Krawczyk-Bärsch
Stable heavy metal collectors in a hostile environment
Even in aqueous solutions with higher uranium contents, magnetotactic bacteria can endure at neutral pH levels. They bind uranium nearly entirely in their cell walls over a wide pH range, which makes them a good choice for coping with the conditions present in mine water. In the procedure, none of the uranium enters the cell’s interior or is bound by the magnetosome.
It was already known that different bacteria, despite possibly having fairly distinct cell structures, could bind heavy metals. In the case of magnetotactic bacteria, the cell walls are made of a thin, four nanometer-thick coating of peptidoglycan, a macromolecule mostly made of sugars and amino acids that is the major component of the cell walls of many bacteria.
The cell walls of magnetotactic bacteria are surrounded by an external membrane composed of sugars and fat-like components: potential docking sites for uranium.
“Our results show that in magnetotactic bacteria peptidoglycan plays the main role in absorbing uranium. This knowledge is new and unexpected in this type of bacteria,” says Krawczyk-Bärsch. The team even managed to identify three specific uranium peptidoglycan species and confirm their findings with reference samples.
Only a combination of microscopy and other spectroscopic techniques, which is uncommon elsewhere in the world, allowed for these novel findings.
“By cooperating with the Institute of Ion Beam Physics and Materials Research at HZDR, for example, we were able to use the electron microscope. The proximity of our institutes at the site and the expertise of our colleagues are a major advantage for our work,” Raff says.
Significance for purifying contaminated water
Thanks to their magnetic properties, magnetotactic bacteria can be easily separated from water using magnets.
“It’s conceivable this could be done on a large scale by carrying out the treatment right in the surface water or by pumping water from underground mines and directing it to pilot treatment plants,” Krawczyk-Bärsch explains.
Because magnetotactic bacteria require little maintenance, they could be a cost-effective substitute for pricy, traditional chemical treatments. Other biomass-based approaches, however, frequently fail because of the high costs associated with increased nutrient and energy requirements.
Researchers’ interest in these bacteria is further inspired by the fact that their proteins can stabilize divalent and trivalent iron, enabling the synthesis of the magnetite contained in the magnetosomes.
“So, we are really asking ourselves how these microorganisms interact with radionuclides in various oxidation states. In particular, we are thinking of plutonium,” explains Raff.
This is due to the possibility that, in contrast to uranium, its chemical resemblance to iron implies it enters the cell by different pathways. How does this affect the way that plutonium migrates in the natural world? Could this also be a method for eliminating plutonium from sewage?
As a result, the subject is pertinent to repository research as well; any findings might then be included in the safety assessment.