The majority of schizophrenia genetics research has sought to understand the role that genes play in the development and heritability of schizophrenia. Many discoveries have been made, but there are still many puzzle pieces missing. Scientists at the University of North Carolina School of Medicine have now conducted the largest-ever whole-genome sequencing study of schizophrenia to provide a more complete picture of the role the human genome plays in this disease.
The study, co-led by senior author Jin Szatkiewicz, Ph.D., associate professor in the UNC Department of Genetics, and published in Nature Communications, suggests that rare structural genetic variants may play a role in schizophrenia.
“Our findings suggest that ultra-rare structural variants that affect the boundaries of a specific genome structure increase the risk of schizophrenia,” said Szatkiewicz. “Alterations in these boundaries may result in dysregulation of gene expression, and we believe that future mechanistic studies will be able to determine the precise functional effects these variants have on biology.”
Scientists have conducted the largest-ever whole-genome sequencing study of schizophrenia to provide a more complete picture of the role the human genome plays in this disease.
Previous studies on the genetics of schizophrenia focused primarily on SNPs (alterations in common genetic sequences affecting a single nucleotide), rare variations in the part of DNA that provides instructions for making proteins, or very large structural variations (alterations affecting a few hundred thousand of nucleotides). These studies provide snapshots of the genome, leaving a large portion of the genome unknown as it may be related to schizophrenia.
Szatkiewicz and colleagues examined the entire genome in the Nature Communications study, employing a technique known as whole-genome sequencing (WGS). The main reason WGS hasn’t been used more widely is that it’s very expensive. An international collaboration pooled funding from National Institute of Mental Health grants and matching funds from Sweden’s SciLife Labs to conduct deep whole-genome sequencing on 1,165 people with schizophrenia and 1,000 controls for this study, which is the largest known WGS study of schizophrenia ever conducted.
New discoveries were made as a result. Previously undetectable DNA mutations in schizophrenia were discovered, which scientists had never seen before.
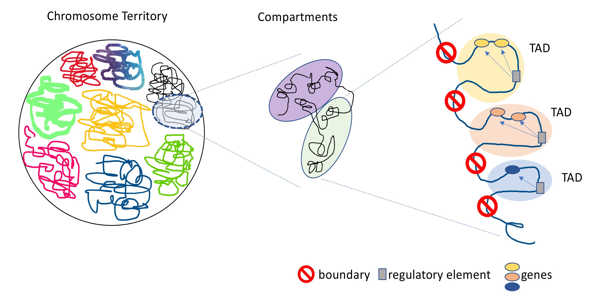
This study focused on the role that topologically associated domains (TADs), a three-dimensional genome structure, may play in the development of schizophrenia. TADs are distinct regions of the genome with strict boundaries that prevent domains from interacting with genetic material from neighboring TADs. Shifting or breaking these boundaries allows interactions between genes and regulatory elements that would not otherwise be possible.
When these interactions occur, gene expression may be altered in unfavorable ways, potentially leading to congenital defects, cancer formation, and developmental disorders. This study discovered that extremely rare structural variants affecting TAD boundaries in the brain are significantly more common in people with schizophrenia than in those who do not have it. Structural variants are large mutations that may involve missing or duplicated genetic sequences, as well as sequences not found in the typical genome.
This finding suggests that misplaced or absent TAD boundaries may also play a role in the development of schizophrenia. This was the first study to discover a link between TAD anomalies and the development of schizophrenia. This research has identified TADs-affecting structural variants as promising candidates for future mechanistic studies of schizophrenia biology.
“A potential future investigation would be to work with patient-derived cells with these TADs-affecting mutations and figure out exactly what happened at the molecular level,” said Szatkiewicz, an adjunct assistant professor of psychiatry at UNC. “In the future, we may be able to use this knowledge of TAD effects to help develop drugs or precision medicine treatments that repair disrupted TADs or affected gene expressions, potentially improving patient outcomes.”
This study will be combined with other WGS studies to increase the sample size and confirm these findings. This study will also aid the scientific community in unraveling the genetic mysteries of schizophrenia.