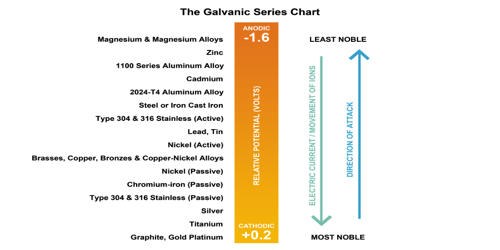
The galvanic series determines the nobility of metals and semi-metals. When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion. When two metals are submerged in an electrolyte, while also electrically connected by a metallic conductor, the less noble will experience galvanic corrosion. The rate of corrosion is determined by the electrolyte, the difference in nobility, and the relative areas of the anode and cathode exposed to the electrolyte.
The galvanic series is a chart showing the relationships and a guide for selecting metals that can be joined, with the aim of helping in the decision-making process. The use of the galvanic series has to be done with caution and basic knowledge of the environments that are a necessary part of this serious form of corrosion. These series determine the electrochemical potential and nobility of metals and metal alloys. It is a list of metal and alloys based on their relative potentials in a specified environment. The environment generally used is seawater.
The characteristics of this series are as follows:
- It indicates the relative positions of metals and alloys instead of potentials.
- The practical measurement of corrosion potential at equilibrium is the basis of arranging the series.
- According to this series, we can connect two alloys without any corrosion.
The galvanic series serves as a simple qualitative guide only and does not give any information on the galvanic corrosion rate. The difference can be measured as a difference in voltage potential: the less noble metal is the one with a lower (that is, more negative) electrode potential than the nobler one, and will function as the anode (electron or anion attractor) within the electrolyte device functioning as described above (a galvanic cell). The anodic metal will corrode in preference to the more passive metal, which becomes cathodic. The galvanic reaction is the principle upon which batteries are based. However, the series does not provide any information on the rate of galvanic corrosion and thus serves as a basic qualitative guide only.
Galvanic series is an arrangement of metals and semi-metals according to their nobility. Nobility is the resistance to corrosion and oxidation in the presence of moist air. The less noble metals become the anode and corrodes faster than it would all by itself, while the other becomes the cathode and corrodes slower than it would alone. This process happens when two metals are submerged in an electrolyte or when electronically connected, before letting the base experience galvanic corrosion. The rate of corrosion on the less noble metal is determined by the electrolyte, the difference in nobility, and the relative areas of the anode and cathode exposed to the electrolyte.