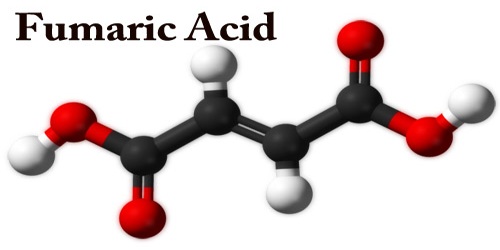
Fumaric acid is an important kind of organic compound with the formula HO2CCH=CHCO2H. It is a dicarboxylic acid, and in the Krebs tricarboxylic acid (TCA) cycle it is a precursor of L-malate. A solid, white fumaric acid is widely found in nature. It has a fruit-like taste and was used as an additive to food. In the meantime, it is also an important type of maleic anhydride derivatives that are widely used in foods, coatings, resins, and plasticizers. Its E number is E297.
The fumaric acid is formed by succinate dehydrogenase oxidizing succinic acid. Fumarate is converted to malate by the enzyme fumarase. The salts and the esters are called fumarates. Fumarate can also refer to the C4H2O2−4 ion (in solution). The Trans isomer has a dipolar moment. Fumaric acid, used as a souring agent in the food industry, can be added to soft drinks, western-style wine, cold beverages, fruit juice concentrate, canned fruit, pickles, and ice cream.
Recently, fumaric acid has been identified as an oncometabolite or an endogenous, metabolite causing cancer. Large levels of this organic acid can be found in tumors or underlying tumors with biofluids. The oncogenic activity tends to be due to its capacity to suppress enzymes including prolyl hydroxylase. This has excellent bubble longevity with delicate product organization, as an acidic material used as a strong beverage gas production agent.
Fumaric acid could be a common artificial additive included in many processed foods to stay them stable and to feature tartness. The substance encompasses a more sour flavor than acid, another common artificial additive. It’s one in all two isomeric unsaturated dicarboxylic acids, the opposite being acid. In acid, the acid groups are Trans (E) and in acid, they’re cis (Z).
Fumaric acid appears as a colorless crystalline solid; the first hazard is that the threat to the environment. It’s found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Iceland moss. As an additive, acid is produced synthetically, mainly from malic acid from apples. It is found that fumaric acid is linked to fumarase deficiency which is an inborn metabolism defect.
Fumarate is an intermediate in the production of citric acid used by the cells to derive energy from food in the form of adenosine triphosphate (ATP). Fumaric acid is odorless with a specific and intense sour, about 1.5 times that of citric acid. Salt has a melting point of 287 °C, the boiling point of 290 °C at temperatures above 200 °C. It will lose water when heated to 230 °C and will become maleic anhydride. DL-malic acid can be formed by co-boiling with water. It is soluble in ethanol and highly soluble in water and ether, but chloroform and insoluble. It is formed by the enzyme succinate dehydrogenase which oxidizes succinate. Then fumarate is converted to malate through the enzyme fumarase.
Fumaric acid is additionally utilized in oral pharmaceutical formulations and food products and is mostly considered a comparatively nontoxic and nonirritant material. It’s generally utilized in beverages and baking powders that requirements are placed on purity. It should be synthesized by the action of certain fungi (Rhizopus nigricans) on glucose; by oxidation of furfural with the salt within the presence of vanadic acid.
Fumaric acid is also used as a mordant for dyes in the manufacture of polyester resins and polyhydrate alcohols. Naturally, fumaric acid occurs in plants such as lichen and Iceland moss and also in animals. By adding fumaric acid to their feed, lambs during digestion produce up to 70 percent less methane.
Information Sources: