Ferrocene is a kind of aromatic-like organic transition metal compound. It is also called Dicyclopentadienyliron and its molecular formula is Fe(C5H5)2. It is uncertain when ferrocene was first created, but process technicians inspecting pipes at a Union Carbide cracker, which was used in the manufacture of the small hydrocarbon cyclopentadiene from dicyclopentadiene, seem to have been reported as a ‘yellow sludge’ in the late 1940s. It is an orange solid that sublimes above room temperature with a camphor-like odor and is soluble in most organic solvents.
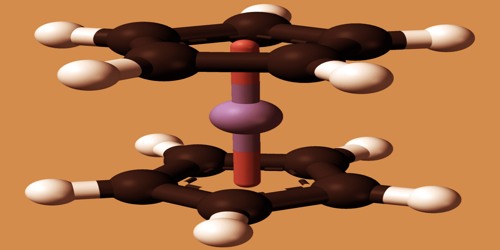
Crystal structure of Ferrocene
For its durability, Ferrocene is remarkable: it is unaffected by air, water, solid bases, and can be heated without decomposition to 400° C. It can reversibly react with strong acids in oxidizing conditions to form the ferrocenium cation Fe(C5H5)+2. In the 1950s, the reaction between cyclopentadienyl magnesium bromide and anhydrous ferric chloride was first successfully developed by people in the last century. It is orange needle crystal powder at room temperature with a smell similar to that of camphor and belongs to a non-polar compound. It is soluble in many organic solvents like methanol, ethanol, divinyl ether, petroleum ether, gasoline, kerosene, diesel oil, dichloromethane, benzene, toluene, and xylene; thanks to the massive polarity of ethanol polarity, it’s usually recommended to dissolve it with toluene.
Ferrocene, with a melting point of 174° C (345° F), occurs as highly stable orange crystals. It can be argued, however, that it was the discovery of ferrocene that initiated organometallic chemistry as a separate field of chemistry. It has also contributed to an explosion of interest in d-block metal compounds with hydrocarbons. Ferrocene owes its unusual stability to the actual fact that when one proton is off from the cyclopentadiene molecule, C5H6, it becomes the flat and aromatic cyclopentadienyl ion, C5H5–.
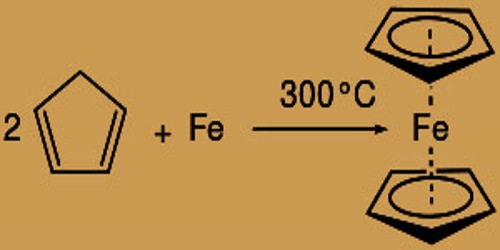
Ferrocene Synthese
Ferrocene is water-insoluble, but condensed sulfuric acid is soluble. It is therefore insoluble in boiling caustic soda solution and hydrochloric acid and does not decompose. With higher thermal stability, chemical stability, and radiation tolerance, this molecule exhibit polarity. It has a wide range of applications in manufacturing, agriculture, medicine, aerospace, energy, protection of the environment, and other sectors. Chemically, in this it undergoes substitution reactions, ferrocene acts as benzene and other aromatic compounds.
However, Below 110 K, ferrocene crystallizes in an orthorhombic space lattice during which the Cp rings are ordered and eclipsed, in order that the molecule has symmetry group D5h. It is often used because of the fuel-saving smoke suppressants and anti-knock agents. It can be used as a catalyst, such as a catalyst for the manufacture of ammonia, as a silicone rubber healing agent; it can avoid light degradation of polyethylene; it can break its natural degradation when applied to agricultural mulch without affecting cultivation and fertilization within a certain period of time.
Ferrocene may also be used as an anti-knock gasoline agent. In order to prevent environmental pollution and poisoning to the human body by a discharge of fuel, it may substitute gasoline toxic tetraethyl lead for being used as the anti-knock agent and for the manufacture of high-grade unleaded petrol. It may be used as radiation absorbers, heat stabilizers, light stabilizers, and smoke-retardants. Ferrocene readily sublimes, especially upon heating during a vacuum. Its vapor pressure is about 1 Pa at 25° C, 10 Pa at 50° C, 100 Pa at 80° C, 1000 Pa at 116° C, and 10,000 Pa (nearly 0.1 atm) at 162° C. The removal of one electron from the molecule lifts the iron atom to the next higher state of oxidation (i.e. from +2 to +3), resulting in the formation of blue ferrocenium cation salts, (C5H5)2Fe+.
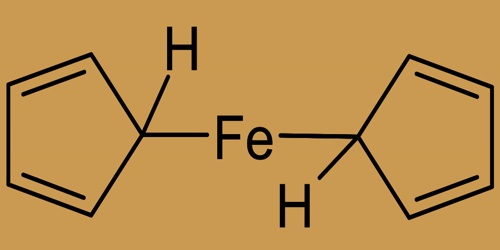
Ferrocene’s molecular structure
Ferrocene undergoes several reactions that are typical of aromatic compounds, allowing substituted derivatives to be prepared. Metallization, acylation, alkylation, sulfonation, formylation, and ligand exchange reaction can also be involved, which can be used for the manufacture of derivatives for a wide variety of applications. It has a good UV-absorbing property and has excellent thermostability that can withstand heating at a temperature as high as 470° C. This interacts with the diffrocenyl-dithiadiphosphetane disulfide formed by P4S10.
Information Sources: