Ferric sulfate (or Iron(III) sulfate) is a grayish-white powder or a yellow crystalline solid. It belongs to the Fe2(SO4)3(H2O)n class of inorganic compounds. The most popular type of “ferric sulfate” is hydrates, which come in a variety of forms. It can be used as a mordant in dyeing and as a coagulant in industrial waste treatment. It may be used as an astringent and styptic in the medical field. The threat to the ecosystem is the most serious danger. To prevent it from spreading to the environment, immediate action should be taken. It’s used as a soil conditioner and for water purification.
Ferric sulfate is a solution made from iron wastes that is commonly used. Although the exact species is unknown, its applications do not necessitate high purity materials. It is a type of hemostatic agent that has the ability to regulate or stop the flow of blood. Ferric sulfate is also recommended as a strong pulpotomy agent in primary teeth, with the ability to replace formocresol. It can also be used as a dye and in aluminum and steel pickling baths. It’s normally made by adding ferrous sulfate, sulfuric acid, and an oxidizing agent like nitric acid in a reaction.
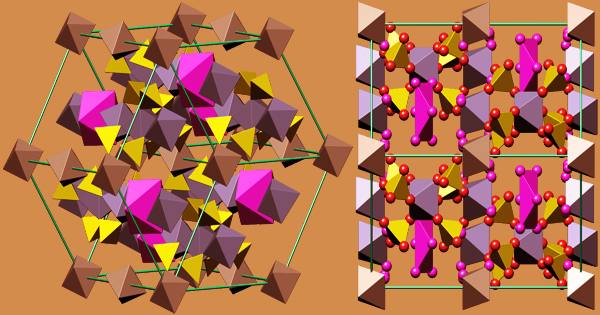
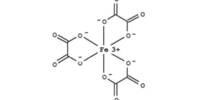
Ferric sulfate is an iron-sulfate compound with a 3:2 ratio of iron(3+) to sulfate ions. It’s a catalyst, a mordant, and an astringent all in one. It consists of a metal sulfate and an iron molecular entity, as well as an iron(3+). Sulfuric acid, a hot solution of ferrous sulfate, and an oxidizing agent are used to make it on a wide scale. Chlorine, nitric acid, and hydrogen peroxide are common oxidizing agents.
2 FeSO4 + H2SO4 + H2O2 → Fe2(SO4)3 + 2 H2O
It also may be prepared by treating iron(III) oxide with sulfuric acid:
Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
Iron sulfates are found in a variety of minerals that are both rare and commercially unimportant. Mikasaite is a mixed iron-aluminum sulfate with the chemical formula (Fe3+, Al3+)2(SO4)3. It is a type of iron(III) sulfate. Grayish-white rhombic crystals of anhydrous salt; hygroscopic; density 3.10 g/cm3; slightly soluble in cold water; decomposes in hot water. The nonahydrate is a yellow hexagonal crystalline material with a refractive index of 1.54, a density of 2.10 g/cm3, and a hardness of 2.5 Mohs. It decomposes at 400°C and is very water soluble.
As pulpotomy agents, ferric sulfate 15.5 percent solution (applied for 15 seconds for 35 teeth) and formocresol solution were used. It is an iron-containing food and dietary supplement. This anhydrous form is only present in very small quantities and is related to coal fires. Hydrates are more common, with coquimbite (nonahydrate) being the most commonly encountered. The other natural nonahydrate is paracoquimbite, which is an unusual find. Rarely are kornelite (heptahydrate) and quenstedtite (decahydrate) found. Lausenite (hexa- or pentahydrate) is a species that has been questioned.
The respiratory tract is irritated when ferric salts are inhaled as dusts or mists. Ferric salts are known to irritate the skin. Forage alfalfa, almonds, nurseries, and structural pest control all use ferric sulfate. Pigments, textile dyeing, water treatment, and metal pickling all use this material. It’s also used in aluminum and steel pickling pools. If the content was not burned, keep it away from water supplies and sewers. As required, create dikes to absorb the flow.
Information Sources: