Electrosynthesis is the synthesis produced by means of an electric current. It is naturally a huge topic within the field of electrochemistry. Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reaction, electrosynthesis sometimes offers improved selectivity and yields. In addition, electrochemistry is mostly taught by physical chemists, which seems to create a natural barrier to preparative organic applications. Electrosynthesis is actively studied as a science and also has industrial applications. Electro-oxidation has the potential for wastewater treatment as well. However, the systematic use of cationic species as intermediates to avoid over-oxidation establishes new ways for the functionalization of substrates and paves the way to novel synthetic tools.
Today’s chemists have been simply reluctant to adopt electrosynthesis, believing the technology is too cumbersome or expensive. Electrochemical synthesis is involved in major industrial processes, such as chlorine generation or aluminum manufacturing. Yet as a growing cadre of researchers is showing, the benefits of the technology can no longer be overlooked.
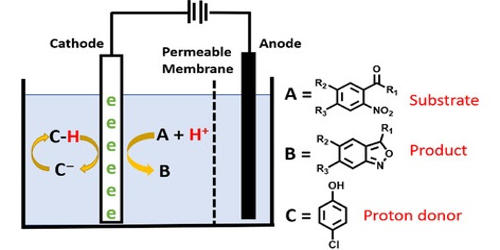
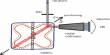
Experimental setup
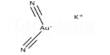
Electrosynthesis in organic chemistry is the synthesis of chemical compounds in an electrochemical cell. The basic setup in electrosynthesis is a galvanic cell, a potentiostat, and two electrodes. Electrosynthesis of organic and inorganic compounds by electrolysis of particular reactants actually employs the FC technology by introducing gas diffusion electrodes (GDEs) in which the gas consumption/evolution reactions take place. Typical solvent and electrolyte combinations minimize electrical resistance. Protic conditions often use alcohol-water or dioxane-water solvent mixtures with an electrolyte such as a soluble salt, acid, or base. his has allowed a significant saving of energy in important industrial processes such as hydrodimerization of acetonitrile and in chlorine/alkali cells. Aprotic conditions often use an organic solvent such as acetonitrile or dichloromethane with electrolytes such as lithium perchlorate or tetrabutylammonium salts. Electrochemical synthesis possesses major benefits. Just like the photons mentioned earlier, electrons are also clean reagents; they do not generate any waste formation. The choice of electrodes with respect to their composition and surface area can be decisive.
For example, in aqueous conditions, the competing reactions in the cell are the formation of oxygen at the anode and hydrogen at the cathode. In this case, a graphite anode and lead cathode could be used effectively because of their high overpotentials for oxygen and hydrogen formation respectively. Many other materials can be used as electrodes. The main advantage of electrosynthesis over an ordinary redox reaction is an avoidance of the potential wasteful other half-reaction and the ability to precisely tune the required potential.
Organic electrosynthesis means synthesizing organic compounds through the use of electricity. In divided cells, the cathode and anode chambers are separated with a semi-porous membrane. Common membrane materials include sintered glass, porous porcelain, polytetrafluoroethylene, or polypropylene. The purpose of the divided cell is to permit the diffusion of ions while restricting the flow of the products and reactants. Usually, electrochemistry is recognized as an environmentally benign tool for organic synthesis.