Principle purpose of this article is to Discuss and Analysis on Faraday’s Laws. The electrochemical cell with zinc as well as copper electrodes had an overall potential difference that seemed to be positive (+1.10 volts), so the spontaneous chemical reactions produced a power current. Such a cell is termed a voltaic cell. In comparison, electrolytic cells use a externally generated electrical current to generate a chemical reaction that wouldn’t normally otherwise take place.
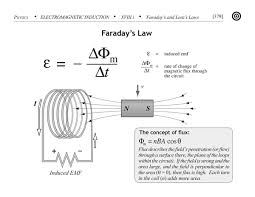
Discuss and Analysis on Faraday’s Laws