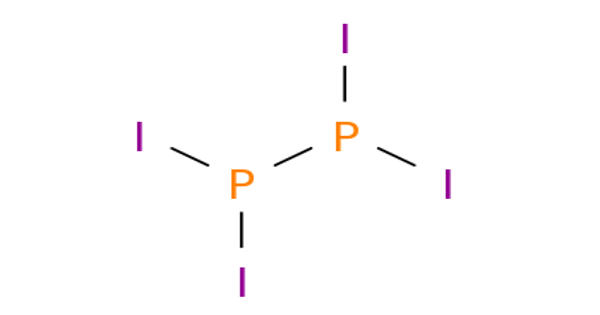
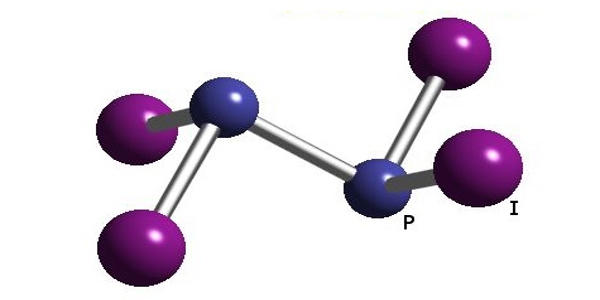
Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It is an orange crystalline solid, and a versatile reducing agent. It has been used as a reducing agent in organic chemistry. It consists of two phosphorus atoms in the oxidation state +2, which via a single bond connected to each other. Each phosphorus atom also has two bonds to iodine atoms. It is made up of phosphorous and iodine which are both non-metals, it is a covalent bond. It is a rare example of a compound with phosphorus in the +2 oxidation state and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.
Properties
Diphosphorus tetraiodide is a covalent compound. It has low melting point as compared to ionic compounds. It is a rare compound where the oxidation state of Phosphorous is +2. It is a solid compound at room temperature that melts at 125.5° C.
- Formula weight: 569.565
- Class: iodide
- Colour: red
- Appearance: solid, triclinic needles
- Melting point: 125.5.
Synthesis and structure
Diphosphorus tetraiodide is easily generated by the disproportionation of phosphorus triiodide in dry ether:
2 PI3 → P2I4 + I2
It can also be obtained by treating phosphorus trichloride and potassium iodide in anhydrous conditions.
The compound adopts a centrosymmetric structure with a P-P bond of 2.230 Å.
Reactions
- Inorganic chemistry
Diphosphorus tetraiodide reacts with bromine to form mixtures PI3−xBrx. With sulfur, it is oxidized to P2S2I4, retaining the P-P bond. It reacts with elemental phosphorus and water to make phosphonium iodide, which is collected via sublimation at 80 °C.
- Organic chemistry
Diphosphorus tetraiodide is used in organic synthesis mainly as a deoxygenating agent. It is used for deprotecting acetals and ketals to aldehydes and ketones, and for converting epoxides into alkenes and aldoximes into nitriles. It can also cyclize 2-aminoalcohols to aziridines and to convert α,β-unsaturated carboxylic acids to α,β-unsaturated bromides.
Applications
- Diphosphorus tetraiodide is used in organic chemistry for converting carboxylic acids to nitriles, for deprotecting acetals and ketals to aldehydes and ketones, and for converting epoxides into alkenes and aldoximes into nitriles.
- It can be used for the synthesis of aziridines from amino alcohols.
- It can also cyclize 2-aminoalcohols to aziridines and to convert α,β-unsaturated carboxylic acids to α,β-unsaturated bromides.
- It is also used to produce nitriles from carboxylic acids.
Information Source: