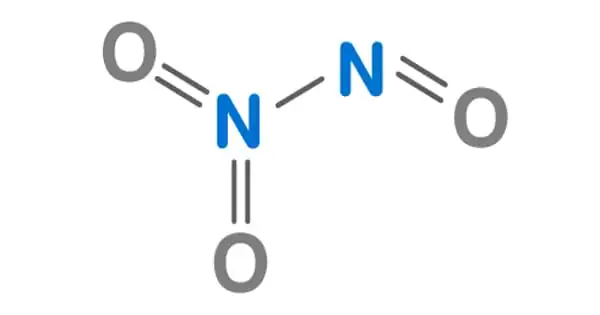
The chemical compound with the formula N2O3 is dinitrogen trioxide. It has the appearance of a blue liquid with a strong, unpleasant chemical odor. It is a deep blue chemical compound created by mixing equal parts nitrogen dioxide and nitric oxide and cooling the liquid below 21 degrees Celsius. It is a type of simple nitrogen oxide. It is a strong oxidant that is poisonous and corrosive.
Dinitrogen Trioxide is the chemical name for a substance produced by mercury and chlorine. Nitrogen sesquioxide is another name for it. It is also a very poisonous natural chemical that is irritating to mucous membranes. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21°C (−6 °F):
NO + NO2 ⇌ N2O3
Only at low temperatures, i.e. in the liquid and solid phases, is dinitrogen trioxide isolable. It has a vivid blue color in both liquid and solid form. With Kdiss = 193 kPa (25 °C), the equilibrium favors the constituent gases at higher temperatures.
Dinitrogen trioxide is a liquid having a strong, disagreeable odor. C-symmetry is exhibited by a planar molecule. This chemical is sometimes referred to as “nitrogen trioxide,” however this term actually refers to another compound, the (uncharged) nitrate radical NO3.
Properties
Its appearance is deep blue-tinted gas and is easily soluble in water. Its boiling point is 3.5-degree C, its melting point is -11.7-degree C and density is 1.4 gram per cubic cm. It is a popular oxidizing agent and is non-flammable but may cause fires when mixed with combustible materials. It reacts with reducing agents to generate heat and products like some gases.
- Appearance: Deep Blue tinted gas
- Boiling Point: 3.5 °C
- Melting Point: −100.7 °C
- Density: 1.4 g/cm³ (liquid) 1.783 g/cm3 (gas)
- Molar mass: 76.01 g/mol
- Solubility in Water: Soluble
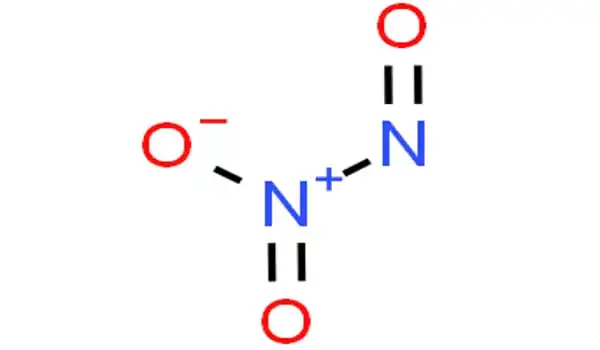
Structure and bonding
N–N bonds are typically similar in length to those found in hydrazine (145 pm). However, dinitrogen trioxide possesses an extremely long N–N bond of 186 pm. Other nitrogen oxides, such as dinitrogen tetroxide, have lengthy N–N bonds (175 pm). The N2O3 molecule has Cs symmetry and is planar.
It is the anhydride of the unstable nitrous acid (HNO2) that is formed when it is combined with water. An alternate structure for the genuine anhydride, namely O=N–O–N=O, might be expected, but this isomer is not seen. If the nitrous acid is not promptly consumed, it decomposes into nitric oxide and nitric acid. Nitrite salts are sometimes formed by adding N2O3 to base solutions:
N2O3 + 2 NaOH → 2 NaNO2 + H2O
Here the oxidation state of one nitrogen is +3.
Uses
Due to its high combustibility, dinitrogen trioxide is employed as a special purpose fuel. The chemical simply aids in combustion and does not itself burn. It is most commonly utilized as an oxidizing agent in conjunction with other chemical substances.