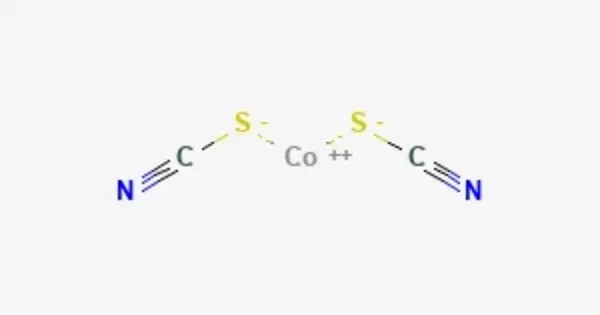
Cobalt(II) thiocyanate is an inorganic compound with the formula Co(SCN)2. It is an inorganic compound featuring cobalt in the +2 oxidation state bonded to thiocyanate (SCN⁻) ligands. The anhydrous compound is a coordination polymer with a layered structure. The trihydrate, Co(SCN)2(H2O)3, is a isothiocyanate complex used in the cobalt thiocyanate test (or Scott test) for detecting cocaine.
It is moderately toxic, requiring careful handling, and can release toxic fumes (e.g., cyanides) upon heating. Its applications extend to catalysis and as a precursor in synthesizing other cobalt compounds. Environmental concerns arise due to cobalt’s potential toxicity, necessitating proper disposal.
Properties
It appears as a pink to red crystalline solid, though it can form blue complexes in certain solutions. With a molar mass of 175.09 g/mol, it is soluble in water, forming a blue solution due to the formation of [Co(SCN)₄]²⁻, a tetrahedral complex, which is often used in qualitative analysis to detect cobalt ions.
- Appearance: It typically appears as a blue or green crystalline solid, depending on its hydration state.
- Chemical formula: C2CoN2S2
- Molar mass: 175.098 g/mol
- Density: 2.484 g/cm3
- Magnetic susceptibility (χ): +11,090·10−6 cm3/mol
Natural Occurrence
Cobalt(II) thiocyanate does not occur naturally in significant quantities. Cobalt is typically found in minerals like cobaltite (CoAsS) or erythrite (Co₃(AsO₄)₂·8H₂O), and thiocyanate is a synthetic ligand derived from cyanide or thiocyanate salts.
Structure and preparation
The structures of Co(SCN)2 and its hydrate Co(SCN)2(H2O)3 have been determined using X-ray crystallography. Co(SCN)2 forms infinite 2D sheets as in the mercury(II) thiocyanate structure type, where as Co(SCN)2(H2O)3 consists of isolated tetrahedral Co(SCN)2(H2O)2 centers and one equivalent of water of crystallization.
The hydrate may be prepared by the salt metathesis reactions, such as the reaction of aqueous cobalt(II) sulfate and barium thiocyanate to produce a barium sulfate precipitate, leaving the hydrate of Co(SCN)2 in solution:
CoSO4 + Ba(SCN)2 → BaSO4 + Co(SCN)2
or the reaction of the hexakisacetonitrile cobalt(II) tetrafluoroborate and potassium thiocyanate, precipitating KBF4
[Co(NCMe)6](BF4)2 + 2KSCN → 2KBF4 + Co(SCN)2.
The anhydrate can then be prepared via addition of diethylether as an antisolvent.
Applications
Cobalt(II) thiocyanate is synthesized by reacting cobalt(II) salts, like cobalt chloride, with alkali thiocyanates. It is commonly used in the Scott test to detect cocaine, where it forms a blue precipitate with the drug. The compound is also utilized in chemical education to demonstrate coordination chemistry and Le Chatelier’s principle, as its color changes with ligand concentration or solvent.
- Analytical chemistry: Used to detect cobalt ions and in tests for certain organic compounds (e.g., cocaine).
- Research: Studied for its coordination chemistry and complex formation.
- Industrial: Limited use, but cobalt compounds are generally used in pigments, catalysts, and battery materials.
Safety Considerations
- Toxicity: Cobalt(II) thiocyanate is toxic if ingested or inhaled, and thiocyanate can release cyanide ions under certain conditions. Handle with care in a well-ventilated area.
- Environmental Impact: Cobalt compounds can be harmful to the environment, and thiocyanate waste requires proper disposal to avoid contamination.