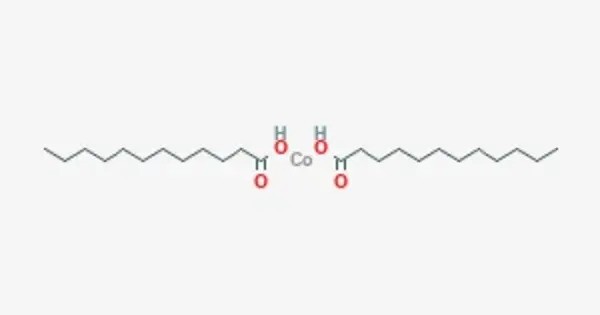
Cobalt laurate is a metal-organic compound with the chemical formula C24H48CoO4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid (lauric acid). It is often used as a drier in paint and varnish formulations. As a drier, it helps to accelerate the drying process of these coatings by promoting the oxidation of oils.
Cobalt laurate can also be used in various industrial applications, such as in the production of catalysts or in the formulation of certain types of polymers.
Synthesis
Cobalt laurate can be prepared by the reaction of aqueous solutions of cobalt(II) chloride (CoCl2) with sodium laurate. It is relatively stable under normal conditions but should be kept away from strong acids or bases, which could potentially react with it.
Properties
Cobalt laurate forms dark violet crystals. It does not dissolve in water, but is soluble in alcohol. It often appears as a dark blue or purple solid, depending on the specific form and purity. It is generally insoluble in water but may dissolve in organic solvents like alcohols or certain oils. Specific melting points can vary, but cobalt salts with fatty acids often have high melting points.
- Chemical formula: C24H48CoO4
- Molar mass: 459.6
- Solubility in water: Insoluble
Applications
- Industrial Applications: Cobalt laurate is used in various industrial applications, including as a drier in paints and coatings. It helps to accelerate the drying process of oil-based paints by promoting oxidation.
- Catalysis: It can act as a catalyst in certain chemical reactions due to the ability of cobalt to facilitate reactions involving organic compounds.
- Chemical Synthesis: It may be used in the synthesis of other cobalt compounds or in research contexts where cobalt’s properties are required.
- Pigments and Dyes: Although less common, cobalt compounds are sometimes used in pigments and dyes, and cobalt laurate might play a role in specific formulations.