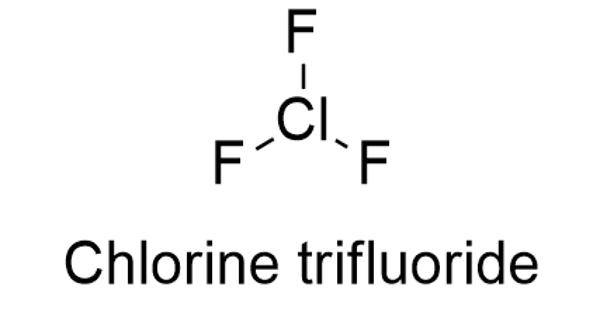
Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. It is an interhalogen compound with the formula ClF3. It is toxic, corrosive, and incredibly reactive with practically every element on the planet. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). The chemical is rather useful as a high-energy fluorinating agent or incendiary material.
Chlorine trifluoride is a chemical compound very unstable, so that it is not found as free compound in nature. The compound is primarily of interest as a component in rocket fuels, in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, and other industrial operations. It is use in electronic industry to clean semiconductors from chemical deposition.
Structure
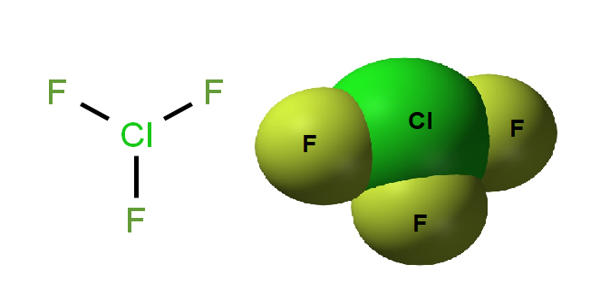
The chlorine fluoride chemical formula is ClF3 and its molar mass is 92.45 g mol-1. The molecule is formed by a cation chloride central which is bound to 3 anions fluoride.
Properties
Chlorine trifluoride is a colorless gas or green to yellow liquid, with pungent odor. Its melting and boiling point are -76 ºC and 12 ºC and its density is 1.77 g mL-1. It is soluble in water, however it decomposes violently in hot water due it reacts to form chlorine and hydrofluoric acid.
Preparation:
There are only a few chemicals that remain completely unreactive with chlorine trifluoride. It was first reported in 1930 by Ruff and Krug who prepared it by fluorination of chlorine; this also produced ClF and the mixture was separated by distillation. Chlorine fluoride is produced by the reaction between chlorine and fluorine gas at 300 ºC, and using a nickel or copper catalyst:
3 F2 + Cl2 → 2 ClF3
The molecular geometry of ClF3 is approximately T-shaped, with one short bond (1.598 Å) and two long bonds (1.698 Å). This structure agrees with the prediction of VSEPR theory, which predicts lone pairs of electrons as occupying two equatorial positions of a hypothetic trigonal bipyramid. The elongated Cl-F axial bonds are consistent with hypervalent bonding.
Pure ClF3 is stable to 180 °C in quartz vessels; above this temperature it decomposes by a free radical mechanism to its constituent elements. Most of chlorine trifluoride is used in nuclear fuel processing.
Hazards
When ClF3 comes into contact with virtually any element, it evaporates into a toxic gas. It is a chemical which should only be handled by professionals. It is a gas or liquid very poisonous to human and environment which is mostly used as rocket propellant especially by the military industry. It is highly corrosive to metals and reacts violently with hot water.
Information Source: