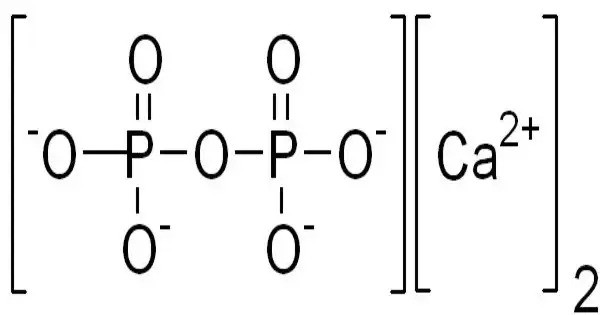Calcium pyrophosphate refers to any member of a series of inorganic compound with the formula Ca2P2O7(H2O)n. It is a calcium salt of pyrophosphate, often found in crystals deposited in joints, leading to a condition known as calcium pyrophosphate deposition disease (CPPD), or pseudogout. They are white solids that are insoluble in water. They contain the pyrophosphate anion, although sometimes they are referred to as phosphates.
Chemically, its formula is Ca₂P₂O₇. These rhomboid-shaped crystals can trigger joint inflammation similar to gout but involve CPP instead of uric acid. Commonly affecting the knees, wrists, and hips, CPPD typically occurs in older adults. It can be idiopathic or associated with conditions like hemochromatosis, hyperparathyroidism, or hypomagnesemia. Diagnosis is confirmed via synovial fluid analysis under polarized light.
Properties
- Chemical formula: Ca2O7P2
- Molar mass: 254.053 g/mol
- Appearance: White powder
- Density: 3.09 g/cm3
- Melting point: 1,353 °C (2,467 °F; 1,626 K)
- Solubility in water: insoluble
- Solubility: soluble in HCl, nitric acids
- Crystal system: Monoclinic (dihydrate)
Chemical Properties
- Stability: Stable under normal conditions, but decomposes at high temperature.
- Hydration forms: Can exist as dihydrate, tetrahydrate, and anhydrous forms, depending on temperature and humidity.
- Insolubility: Insoluble in water, but may dissolve in acidic solutions.
- Reactivity: Can react with acids to form phosphoric acid and soluble calcium salts.
Preparation
Crystals of the tetrahydrate can be prepared by treating a solution of sodium pyrophosphate with calcium nitrate with careful control of pH and temperature:
Na4P2O7(aq)+2 Ca(NO3)2(aq)→ Ca2P2O7·4 H2O + 4 NaNO3
The dihydrate, sometimes termed CPPD, can be formed by the reaction of pyrophosphoric acid with calcium chloride:
CaCl2 + H4P2O7(aq) → Ca2P2O7·2 H2O + HCl.
The anhydrous forms can be prepared by heating dicalcium phosphate:
2 CaHPO4 → Ca2P2O7 + H2O
At 240-500 °C an amorphous phase is formed, heating to 750 °C forms β-Ca2P2O7, heating to 1140 – 1350 °C forms the α-Ca2P2O7.
Application
Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes because of its insolubility and nonreactivity toward fluoride.