The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. It is a key component of bones and teeth, giving them strength and structure. Some so-called calcium phosphates contain oxide and hydroxide as well. Naturally occurring in the body, it is also used in dental products, nutritional supplements, and fertilizers.
Calcium phosphates are white solids of nutritional value and are found in many living organisms, e.g., bone mineral and tooth enamel. It is biocompatible, making it valuable in medical implants and bone grafts. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals, which often are not white owing to impurities, are used in the production of phosphoric acid and fertilizers.
Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals). It’s generally insoluble in water but dissolves in acids. Excess intake can lead to kidney stones or calcium imbalance. Industrially, it’s synthesized from phosphate rocks and is vital in agriculture and food fortification.
Properties
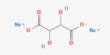
- Chemical formula: Ca3(PO4)2
- Molar mass: 310.18 g/mol
- Appearance: White Solid
- Odor: Odorless
- Density: 3.14 g/cu cm
- Melting point: 1,670 °C (3,040 °F; 1,940 K)
- Solubility in water: Practically insoluble with water
- Solubility in Ethanol: Insoluble with ethanol (also acetic acid)
Chemical Properties
- Reacts with acids to release phosphoric acid and calcium salts.
- Insoluble in ethanol.
- Stable at room temperature, but can decompose at high temperatures.
Biological Significance
- Major component of bones and teeth in the form of hydroxyapatite.
- Biocompatible and bioactive – used in dental and orthopedic implants.
Natural Sources
- Biological: Found in vertebrate bones, teeth, and shells.
- Minerals: Apatite group (mainly fluorapatite, chlorapatite, and hydroxyapatite) – primary source of phosphate rock.
- Occurs in sedimentary rocks, particularly phosphate-rich beds.
Geological Occurrence
Found in phosphate rock deposits, mainly in:
- Morocco (largest reserves)
- China
- United States (Florida, Idaho)
- Russia
Synthetic Production
Produced industrially by reacting calcium hydroxide with phosphoric acid or by the thermal processing of phosphate rock.
Applications
- Fertilizers: Superphosphate, triple superphosphate
- Food additive: Leavening agent (E341)
- Medicine: Calcium supplements, bone grafts
- Dental care: Toothpastes and remineralizing agents
- Ceramics and biomaterials: Used in orthopedic and dental implants